Kim, Jung, Lee, Choi, Chung, and Kang: Comparison of the Quantulus 1220 and 300SL Liquid Scintillation Counters for the Analysis of 222Rn in Groundwater
Abstract
Background
Liquid scintillation counters (LSCs) are commonly used as an analytical method for detecting 222Rn in groundwater because they involve a simple sample pretreatment and allow high throughout with an autosampler. The Quantulus 1220 is the best-selling LSC in Korea, but its production was stopped. Recently, a new type of LSC, the 300SL, was introduced. In this study, the 300SL was compared with the Quantulus 1220 in order to evaluate the ability of each apparatus to detect 222Rn in groundwater.
Materials and Methods
The Quantulus 1220 and 300SL were used to detect the presence of 222Rn. Radon gas was extracted from a groundwater sample using a water-immiscible cocktail in a LSC vial. The optimal analytical conditions for each LSC were determined using a 222Rn calibration source prepared with a 226Ra source.
Results and Discussion
The optimal pulse shape analysis level for alpha and beta separation was 80 for the Quantulus 1220, and the corresponding pulse length index was 12 in the 300SL. The counting efficiency of the Quantulus 1220 for alpha emissions was similar to that of the 300SL, but the background count rate of the Quantulus 1220 was 10 times lower than that of the 300SL. The minimum detectable activity of the Quantulus 1220 was 0.08 Bq┬ĘLŌłÆ1, while that of the 300SL was 0.20 Bq┬ĘLŌłÆ1. The analytical results regarding 222Rn in groundwater were less than 10% different between these LSCs.
Conclusion
The 300SL is an LSC that is comparable to the Quantulus 1220 for detecting 222Rn in groundwater. Both LSCs can be applied to determine the levels of 222Rn in groundwater under the management of the Ministry of Environment.
Keywords: Liquid scintillation counter, Radon, Groundwater, Quantulus 1220, 300SL
Introduction
The annual dose of natural background radiation for an average individual is 2.4 mSv, of which approximately 54% is contributed by 222Rn [ 1, 2]. In the uranium decay series, the daughter nuclide of 226Ra, 222Rn (T 1/2=3.8 days), is known as radon. In the thorium decay series, the daughter nuclide of 224Ra, 220Rn (T 1/2=55 s), is known as thoron and must be distinguished from 222Rn. The decay of radon generates alpha nuclides such as 218Po (T 1/2=3.1 minutes) and 214Po (T 1/2=162 s). The World Health Organization has reported radon to be a main cause of lung cancer and has emphasized the risk posed by radon [ 1, 2]. The U.S. Environmental Protection Agency (EPA) proposed a limit of radon concentration in drinking water of 148 Bq┬ĘL ŌłÆ1 (4,000 pCi┬ĘL ŌłÆ1) [ 3]. Ninety percent of the radon absorbed through drinking water is absorbed through inhalation, not ingestion [ 3]. Based on such reports, the concentration of radon in drinking water is regulated based on the degree to which drinking water contributes to the concentration of radon in indoor air. For example, 4,000 pCi┬ĘL ŌłÆ1 of radon in drinking water converted into the indoor air concentration is 0.4 pCi┬ĘL ŌłÆ1, which corresponds to 10% of 4 pCi┬ĘL ŌłÆ1, the concentration of radon in indoor air set by EPA regulations. Currently, EPA regulations set the concentration of radon in drinking water as 4,000 pCi┬ĘL ŌłÆ1 for regions that implement radon reduction in their drinking water programs and as 300 pCi┬ĘL ŌłÆ1 for regions that do not [ 3]. The Ministry of Environment currently applies the U.S. standard of 4,000 pCi┬ĘL ŌłÆ1 for radon in drinking water to manage the concentration of radon in groundwater. Following the 2007 plan of the third fact-finding survey of naturally occurring radioactive material in groundwater, the Ministry of Environment has been carrying out research on naturally occurring radioactive material, targeting around 300 sites per year through 2016, and has included radon as a substance to monitor [ 4]. The analysis of radon in groundwater can be conducted using radiometric methods such as ion chambers or liquid scintillation counters (LSCs). Analytical equipment incorporating ionization chambers include Alphaguard (Saphymo, Frankfurt, Germany) or RAD-7 (Durridge, Boston, MA). They have the advantage of allowing field analysis using water-kit equipment, which elutes radon gas in groundwater. However, because these products cannot analyze multiple samples at once, they have a smaller sample throughput than LSCs. In addition, LSCs have a low background count rate and a low minimum detectable activity, and involve a simple sample pretreatment method. Moreover, since LSCs are equipped with an autosampler, they can simultaneously handle a large quantity of samples. In South Korea, the analysis of radon in groundwater using LSCs has been universalized. Most studies have used the Quantulus 1220 [ 5ŌĆō 8], but another study used photoelectron-rejecting alpha liquid scintillation (PERALS) [ 9]. The Quantulus 1220 is the most common LSC in South Korea, with around 50 in operation. 1) Currently, 15 300SL LSCs based on the triple-to-double count ratio have been supplied to South Korea. 2) This study conducted a comparative assessment of the use of these 2 LSCs to detect radon in groundwater in South Korea. The analytical conditions of each LSC were tested, and the background count rate and counting efficiency were compared. An assessment of the analytical results depending on the LSC vial type was also carried out. Finally, the results of the Quantulus 1220 and the 300SL regarding radon in groundwater were compared.
Materials and Methods
1. Reagents and instruments
Maxilight (Hidex, Turku, Finland) based on di-isopropyl naphthalene (DIN) was used as the scintillation cocktail to extract radon gas from samples. Maxilight is a water-immiscible scintillation cocktail, so only radon gas and its daughter nuclides are included in the scintillation cocktail [ 10]. The 226Ra radioactive standard source (SRM 4966A) from the National Institute of Standard and Technology was used to prepare the 222Rn calibration source. Plastic vials (Wheaton, Millville, NJ), glass vials (Wheaton, Millville, NJ), and Teflon vials (Flon Chemical, Osaka, Japan) were used as LSC vials, each with a capacity of 20 mL. For the 222Rn analysis, the Quantulus 1220 (Perkin Elmer, Waltham, MA) and 300SL (Hidex, Turku, Finland) were used. Like the Quantulus 1220, the 300SL is equipped with a cooling function and an alpha/beta separation function.
2. Preparation of the 222Rn calibration source
To prepare the 222Rn calibration source, the 226Ra radioactive standard source of 1 Bq was added to a LSC vial and mixed with deionized water until the final volume reached 10 mL. After mixing with 10 mL of the Maxilight scintillation cocktail, we waited one month until 226Ra reached radioactive equilibrium with 222Rn. The 222Rn calibration source was used to determine the counting efficiency and the optimal conditions for alpha/beta separation. Equal 10-mL samples of deionized water and Maxilight were put into an LSC vial and used as a background sample. A 1:1 ratio between the sample and scintillation cocktail showed a high counting efficiency, low background count rate, and low minimum detectable activity (MDA).
3. Groundwater sampling
Sampling is the most important factor when analyzing radon in groundwater. Since radon is dissolved in groundwater in gas form, radon instantly escapes from the sample when the sample is exposed to the atmosphere. If researchers are not attentive during the sampling process, the results could underestimate the actual concentration of radon. After installing a tube that supplies groundwater at the base of a 1-L or larger container, approximately 3 to 4 times as much groundwater as the container volume was pumped through. Using a pipette, 10 mL was extracted from the bottom-most part of the container possible.
Results and Discussion
1. Hardware comparison of the Quantulus 1220 and 300SL
The Quantulus 1220 and 300SL are very different in terms of hardware. The Quantulus 1220 has a width of 101 cm, a depth of 92 cm, a height of 156 cm, and a weight of approximately 1 ton. In contrast, the 300SL has a width of 52 cm, a depth of 63 cm, a height of 68 cm, and a weight of 130 kg, making it possible to use on top of a desk. Its compact weight and size make the 300SL easily transportable, while the Quantulus 1220 is utilized as a fixed detector. The Quantulus 1220 is heavy due to the 750 kg of lead installed in it to shield against external radiation. Less than 10% of the amount of lead in the Quantulus 1220 is installed in the 300SL. This results in a difference in the background count rate. The Quantulus 1220 has a beta background count rate of 3.0┬▒0.2 cpm (mean ┬▒ standard deviation) while the value is more than 10 times greater for the 300SL, at 43.7┬▒2.8 cpm (based on the use of a plastic vial; Ultima Gold AB 10 mL).
As shown in Figure 1, the Quantulus 1220 uses two photon multiplier tubes (PMTs) as detectors, while the 300SL uses three PMTs. The Quantulus 1220 uses a guard detector and an anticoincidence system to reduce the effect of external radiation. For the 300SL, the operator can decide whether to operate a guard detector before analysis, and can reduce the background count rate by 20% if the guard detector is used. For alpha nuclide analysis, the counting efficiency of the 300SL, which operates three PMTs, shows a higher efficiency within the 10% range than the Quantulus 1220, which uses two PMTs ( Table 1). This is because the photoelectrons that are not detected in a PMT composed of 180-degree intervals can be detected in a PMT composed of 120-degree intervals ( Figure 1B and 1E). One advantage of LSCs is that they contain an autosampler, which is a useful function for routine analysis. With the use of a 20-mL LSC vial, the Quantulus 1220 can simultaneously analyze 60 samples, and the 300SL can simultaneously analyze 40 samples.
2. Optimal analytical conditions for the LSCs
226Ra decays while generating 222Rn, 218Po, and 214Po alpha nuclides. Of these products, 218Po and 214Po have short half-lives; they reach radioactive equilibrium with the mother nuclide 222Rn after approximately 3 hours. When considering the near 100% alpha emitter counting efficiency for LSCs, the 222Rn counting efficiency (taking into account 222Rn, 218Po, and 214Po) can theoretically reach 300% in this study, which used the radon evaporation method [ 10]. This is because 222Rn, 218Po, and 214Po form a radioactive equilibrium in the scintillation cocktail in which only radon was extracted. Since 226Ra exists in parts of an aqueous solution, not a scintillation cocktail, its effect on the alpha count rate is so small that it can be disregarded. The alpha count rate is equal to or less than 0.05 cpm (n=5) when 5 Bq of 226Ra is placed into a 20-mL plastic vial, 10 mL of it is filled with 0.1M HNO 3, and the combination is then analyzed using the Quantulus 1220. Based on the LSC analytical results of the 222Rn source, the counting efficiency (╔ø) was determined by Equation 1.
The LSCs used in this study have an alpha/beta separation function. The Quantulus 1220 separates alpha nuclides and beta nuclides based on pulse shape analysis (PSA), while the 300SL separates them based on the pulse length index (PLI). Since the shapes of electrons generated by photons emitted from alpha and beta particles are different, alpha particles trigger a pulse characterized by delayed phosphorescence and beta particles trigger a pulse involving prompt fluorescence. The criterion for distinguishing between alpha and beta nuclides after measuring the pulse decay time or pulse length that occurs at different times is referred to as PSA or the PLI. 241Am and 36Cl sources can be used to determine alpha/beta separation conditions [ 5]. It is important to assess beta spillover (when alpha rays are misidentified and measured as beta rays) and to assess alpha spillover, the opposite phenomenon. Simultaneous alpha/beta analysis must take alpha spillover and beta spillover into account in order to measure accurate levels of alpha and beta emitters. In such cases, the optimal PSA level for separating alpha/beta nuclides is determined when the alpha spillover and beta spillover are at a minimumŌĆöin other words, the optimal PSA level is chosen at the point where the alpha count rate of 241Am reaches its maximum and where, at the same time, the beta count rate of 36Cl reaches its maximum [ 5]. The optimal PSA level of the Quantulus 1220 used in this study was determined based on the figure of merit (FOM) value of the 222Rn calibration source according to the PSA level. Since the 222Rn calibration source includes alpha daughter nuclides ( 218Po, 214Po) and beta daughter nuclides ( 214Pb, 214Bi), the count rate of alpha emitters reflects alpha spillover and beta spillover. Therefore, the higher the count rate in the alpha ray domain, the lower the alpha spillover and beta spillover. The FOM value increases with a lower background count rate and a higher counting efficiency ( Equation 2). The 222Rn source and background sample were measured within the PSA 30 range and the 130 range, respectively. A PSA value of 80, with the maximum FOM value, was identified as the optimal alpha/beta analytical condition [ 10]. The 300SL has a simpler process for determining alpha/beta separation conditions than the Quantulus 1220. After measuring the 222Rn calibration source, the criterion for alpha/beta separation (PLI) is determined by using software. As shown in Figure 2, the analytical results of the 222Rn calibration source can be shown as alpha rays and beta rays in 3-dimensional graph form based on the PLI criterion. Compared to the prompt fluorescence that originates from beta particles, the length of the pulse due to the delayed phosphorescence resulting from alpha particles that react with the scintillation cocktail is longer. The 300SL can visually separate alpha emitters and beta emitters that include pulse length information, and can perform an alpha/beta separation based on a single measurement. In this study, the analysis was carried out based on a PLI of 12, and the counting efficiency and background count rate were determined accordingly.
3. Region of interest set-up
The region of interest (ROI) is another important analytical factor. Since the background count rate and counting efficiency are closely related to the MDA, it is desirable to set an interval with a low background count rate and a high counting efficiency as the ROI. Figure 3 presents the alpha spectrum of the 222Rn calibration source generated using the Quantulus 1220 and 300SL. Table 1 presents the background count rate and counting efficiency of the Quantulus 1220 and 300SL in the 700ŌĆō950 channel condition and the total channel condition. Compared to the analytical results of the total channel, the counting efficiency in the ROI condition was similar, at 230%-245%, but the background count rate decreased from 0.20 cpm to 0.01 cpm in the Quantulus 1220. Compared to the total channel set-up, when a ROI is set, the MDA can be lowered threefold for the same condition (sample volume and measuring time) using the Quantulus 1220.
4. Selection of vial types
In a previous study, plastic vials and glass vials were used to analyze groundwater with a high concentration of radon. When a toluene series scintillation cocktail was used, radon gas leakage was observed after the sample was stored in a vial. To avoid this phenomenon, Teflon vials were recommended [ 5]. This is because the toluene series scintillation cocktail permeated into the plastic vial and was volatilized outside of the container, causing some radon gas to leak as well. When a Maxilight scintillation cocktail was used, no radon gas leakage was observed from either plastic vials or glass vials. In each vial, 10 mL of groundwater was mixed with 10 mL of the Maxilight scintillation cocktail. The first analytical result used the LSC measurement as C 0 and the count rate measured after a certain amount of time as C t ( Equation 3). Based on C 0 (without considering the counting efficiency), the calculated count rate is shown in Figure 4, which presents the measured count rate using the LSC and the 222Rn decay rate. The measured value was strongly correlated with the calculated value. This means that no other reduction factors were present in addition to radiochemical radon decay in plastic or glass vials. When a DIN-series scintillation cocktail is used, it is not necessary to take radon leakage into account, even if expensive Teflon vials are not used.
C0: count rate at the first measurement Cm: count rate at the time with interval from the first measurement ╬╗Rn222: decay rate of 222Rn (dŌłÆ1) tmŌåÆ0: time interval between the measurement (Ct) and the first measurement (C0) (d)
Using the Quantulus 1220, the counting efficiency results of the 222Rn calibration source prepared in a glass vial, a plastic vial, and Teflon vial were 239%┬▒3%, 235%┬▒2%, and 236%┬▒3%, respectively. The counting efficiency difference was not large among the vial types. However, the background count rate results of the glass vial and plastic vial were 0.01 cpm and 0.03 cpm, respectively, showing a relatively low background count rate for the glass vial.
5. Minimum detectable activity
The main variables that determine the MDA are sample quantity, counting time, background count rate, and counting efficiency. The counting efficiency values of Quantulus 1220 and 300 SL are similar, within a range of 10%. Under the ROI condition, the background count rate of Quantulus 1220 is 10 times lower than that of 300SL. When the sample volume is 10 mL and the counting time is 60 minutes, the MDAs of the Quantulus 1220 and 300SL are 0.08 Bq┬ĘL ŌłÆ1 and 0.20 Bq┬ĘL ŌłÆ1, respectively. While the MDA of 300SL is relatively high, it corresponds to 0.1% of 148 Bq┬ĘL ŌłÆ1, the concentration of radon in drinking water suggested by EPA regulations. The MDA was calculated by the following equation [ 11].
6. Application to groundwater samples
To analyze actual radon in groundwater, a glass vial containing 10 mL of the Maxilight scintillation cocktail was prepared. As described in section 2.3, 10 mL of groundwater was extracted on site, put into a vial, and intensely mixed. To avoid sample exposure to the atmosphere, the pipette was placed at the bottom of the vial containing the scintillation cocktail to inject the sample. Analysis was conducted using a LSC after approximately 3 h, when 222Rn reached radioactive equilibrium with 218Po and 214Po. Table 2 presents the results of the analyses conducted with the Quantulus 1220 and 300SL. The counting efficiency and background count rate suitable for each piece of equipment were used, and the radon concentration was calculated using Equation 5.
A: activity concentration of 222Rn (Bq┬ĘLŌłÆ1) Nnet: net count rate (cps) ╔ø: counting efficiency f: ingrowth factor of 222Rn Vsmp: volume of sample (L)
The relative error was calculated based on the results of the Quantulus 1220, and the difference between the radon concentrations of the two LSCs matched within 10%.
Conclusion
The following are the advantages of analyzing radon in groundwater using a LSC: (1) no chemical separation is necessary, (2) the MDA is very low due to the low background count rate, and (3) many samples can be simultaneously processed using an autosampler. The concentration of radon in groundwater was analyzed using the widely distributed Quantulus 1220 and the recently launched 300SL. While the 2 pieces of equipment showed similar functionality in terms of counting efficiency, the background count rate of the Quantulus 1220 was approximately 10 times lower than that of the 300SL. Under the same sample volume and counting time conditions, the MDA of the Quantulus 1220 was 3 times lower than that of the 300SL. With a sample volume of 10 mL and 60 minutes of counting time, the 300SL could detect the concentration of radon in drinking water set forth by EPA regulations (148 Bq┬ĘLŌłÆ1). Radon leakage from plastic or glass vials can be prevented if a DIN series Maxilight scintillation cocktail is used instead of a toluene series cocktail. Since the background count rate is lower when using a glass vial than when using a plastic vial, it is more advantageous to use a glass vial.
Acknowledgments
This study was funded by the Nuclear Research Development Project of the Ministry of Science, ICT and Future Planning (Project Number: 2012M2A8A4025915).
References
1. United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation. UNSCEAR 2000. 2000;97-108.  2. World Health Organization. WHO Handbook on indoor radon: A Public Health perspective. 2009;3-14.
3. United States Environmental Protection Agency. Report to Congress: Radon in drinking water regulations. EPA 815-R-12-002. 2012;1-6.
4. National Institute of Environmental Research. An Investigation on Natural radioactivity Levels in Groundwater (ŌĆ▓10). NIER No. 2010-47-1222. 2010;1-17.
5. Kim CK, Kim CS. A Rapid method for the measurement of 222Rn in groundwater and hot spring water using ultra low-level liquid scintillation counter and pulse shape analysis. J Radiat Prot Res. 1995;20(20):103-115.
6. Kim Y, Cho SY, Yoon YY, Lee KY. Optimal method of radon analysis in groundwater using ultra low-level liquid scintillation counter. J Soil Groundwater Environ. 2006;11(5):59-66.
7. Noh HJ, Jeong DH, Yoon JK, Kim MS, Ju BK, Jeon SH, Kim TS. Natural reduction characteristics of radon in drinking groundwater. J Soil Groundwater Environ. 2011;16(1):12-18.  8. Cho JS, Lee HM, Kim SW, Kim JS. A study on the variation of 222Rn concentration in groundwater at Busan-Geumjeong area. J Radiat Prot Res. 2012;37(3):149-158.  9. Woo HJ, Yoon YY, Cho SY, Chun SK. A study of 222Rn and 226Ra analysis in the groundwater by LSC. J Radiat Prot Res. 1995;20(4):275-283.
10. Jung Y, Kim H, Chung KH, Kang MJ. Study of the determination of 226Ra in soil using liquid scintillation counter. Anal Sci Technol. 2016;29(2):65-72.  11. Currie LA. Limits for qualitative detection and quantitative determination: application to radiochemistry. Anal Chem. 1968;40:586-593. 
Fig.┬Ā1
Front view of (A) the Quantulus 1220, (B) schematic diagram, and (C) photo of the detector (Quantulus 1220); (D) front view of the 300SL, (E) schematic diagram and (F) photo of the detector (300SL). 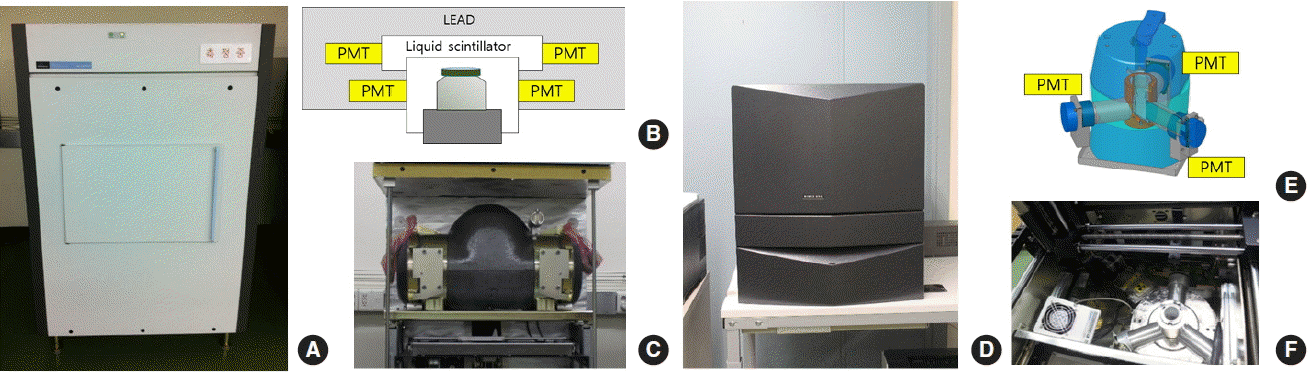
Fig.┬Ā2
Three-dimensional graph of the 222Rn calibration source using the 300SL. 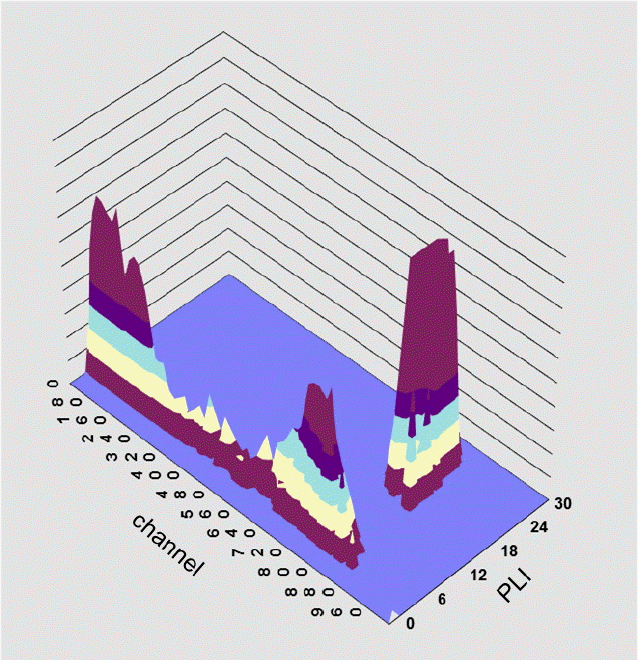
Fig.┬Ā3
Alpha spectrum of the 222Rn calibration source in a glass vial using the Quantulus 1220 (red line) and 300SL (blue line). 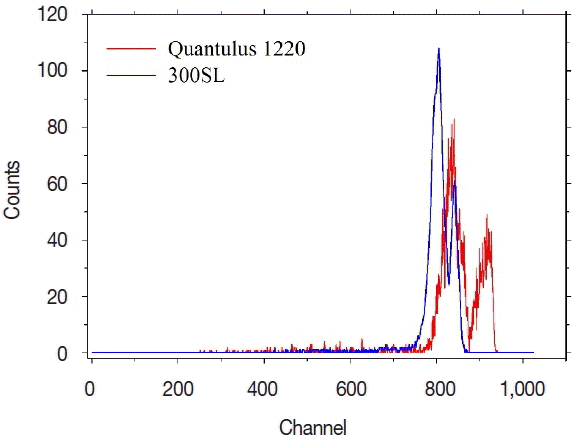
Fig.┬Ā4
Test of loss of 222Rn according to the type of vial (A: plastic vial, B: glass vial). 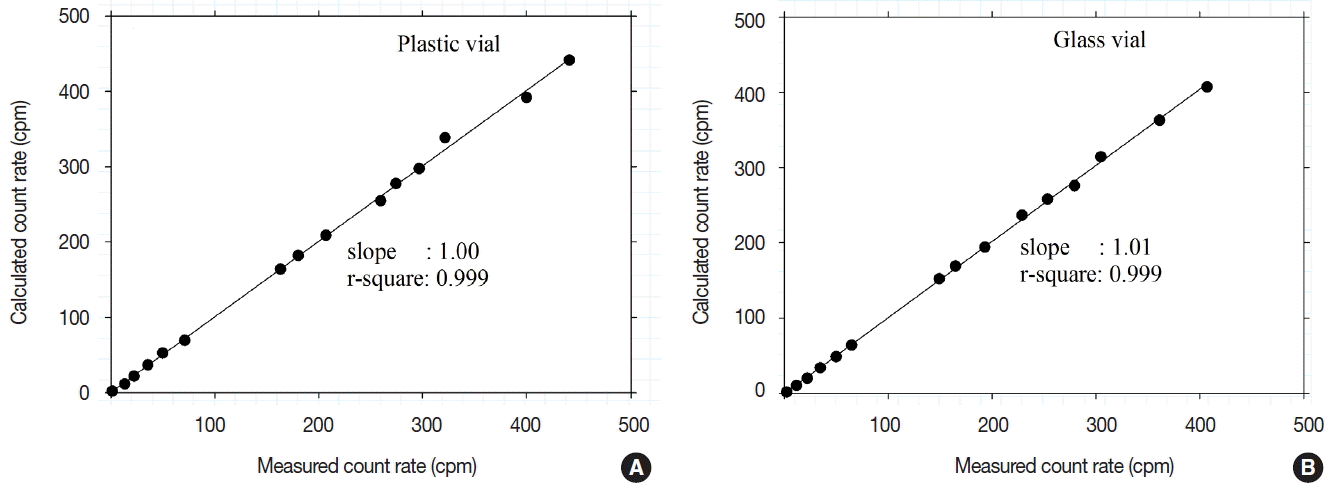
Table┬Ā1
Analytical Results of a Blank Sample using the Quantulus 1220 and the 300SL with Various Channel Ranges
|
Channels 1ŌĆō1024 |
Channels 700ŌĆō950 |
|
|
|
|
Quantulus 1220 |
300SL |
Quantulus 1220 |
300SL |
|
Blank count rate (cpm) |
0.20 |
0.28 |
0.01 |
0.17 |
|
|
Counting efficiency (%) |
239 |
245 |
230 |
238 |
Table┬Ā2
Analytical Results of 222Rn in Groundwater.
|
Sample code |
222Rn (Bq┬ĘLŌłÆ1)*
|
R.E. (%)ŌĆĀ
|
|
Quantulus 1220 |
300SL |
|
S-01 |
39.2┬▒1.5 |
38.5┬▒1.3 |
ŌłÆ2 |
|
S-02 |
19.4┬▒1.0 |
17.6┬▒1.2 |
ŌłÆ9 |
|
S-03 |
26.3┬▒1.1 |
27.5┬▒1.6 |
5 |
|
S-04 |
52.2┬▒1.7 |
53.1┬▒1.2 |
2 |
|
S-05 |
70.7┬▒2.5 |
72.1┬▒2.1 |
2 |
|
|









