AbstractBackgroundThe purpose of this study is to assign an appropriate density to virtual phantom for 2D diode array detector with different dose calculation algorithms to guarantee the accuracy of patient-specific QA.
Materials and MethodsTen VMAT plans with 6 MV photon beam and ten VMAT plans with 15 MV photon beam were selected retrospectively. The computed tomography (CT) images of MapCHECK2 with MapPHAN were acquired to design the virtual phantom images. For all plans, dose distributions were calculated for the virtual phantoms with four different materials by AAA and AXB algorithms. The four materials were polystyrene, 455 HU, Jursinic phantom, and PVC. Passing rates for several gamma criteria were calculated by comparing the measured dose distribution with calculated dose distributions of four materials.
Results and DiscussionFor validation of AXB modeling in clinic, the mean percentages of agreement in the cases of dose difference criteria of 1.0% and 2.0% for 6 MV were 97.2%±2.3%, and 99.4%±1.1%, respectively while those for 15 MV were 98.5%±0.85% and 99.8%±0.2%, respectively. In the case of 2%/2 mm, all mean passing rates were more than 96.0% and 97.2% for 6 MV and 15 MV, respectively, regardless of the virtual phantoms of different materials and dose calculation algorithms. The passing rates in all criteria slightly increased for AXB as well as AAA when using 455 HU rather than polystyrene.
IntroductionVolumetric modulated arc therapy (VMAT) modulating the treatment parameters which are gantry speed, multi-leaf collimator (MLC), and dose rate simultaneously is a novel technique in the field of radiation therapy [1–3]. For this reason, VMAT has been gradually used in the clinic and the prescription doses could be delivered to target volumes with sparing normal tissues with similar plan quality and monitor unit (MU) effectiveness, compared to intensity modulated radiation therapy (IMRT) [4–6]. For VMAT, it has been demonstrated that mechanical uncertainties for driving the multiple modulating parameters and inaccurate dose calculation for small or irregular field in VMAT could make discrepancies between dose distribution calculated in treatment planning system (TPS), and actual dose distribution delivered to the patient [4, 7, 8]. In order to verify a deliverability of VMAT plans, patient-specific quality assurance (QA) should be conducted before starting first fraction of treatment. The most widely used method for patient-specific QA is gamma index method with a planar dose distribution using 2D array detector [9, 10]. Using a combined dose difference (DD) and distance-to-agreements (DTA) criteria, gamma value of at least 1 indicates a failing region, whereas a gamma value less than 1 indicates a passing region [11]. For passing rate which means the portion of passed measurement point according to all calculated gamma index matrix, many clinics have widely used action level of 90% using 3%/3 mm or 2%/2 mm with threshold level of 10%, which were proposed by the American Association of Physicists in Medicine (AAPM) task group (TG) 119 report as a default standard [12, 13].
A verification plan or QA plan is generated and recalculated on the computed tomography (CT) images of 2D array detector equipped with water equivalent phantom in order to compare measured and planned dose distributions. It has been reported that ion chambers and diodes inside 2D array detectors result in artifact of CT images and generate inaccurate Hounsfield Unit (HU) [14, 15]. For these reasons, manufacturers of 2D array detector have provided the virtual phantom design and recommended density or HU value for 2D array detector commissioning [15].
Recently, Acuros XB dose calculation algorithm (AXB, Varian Medical Systems, Palo Alto, CA) has been released as a clinical dose algorithm in Eclipse TPS to achieve both accuracy and speed of dose calculation [16, 17]. It has been reported that AXB calculated dose distribution based on the mass density while anisotropic analytical algorithm (AAA, Varian Medical Systems, Palo Alto, CA) used the electron density for reflecting the characteristics of materials. Our institution has recently commissioned AXB (version 10.0) by comparing the calculated percentage depth dose (PDD) and profiles with the measured those in water phantom. The spot size parameters of AXB were 1.5 mm and 1.0 mm (in both x and y directions) for 6 MV and 15 MV, respectively. The accuracy of patient-specific QA is directly related to dose calculation algorithm as well as detector. The purpose of this study is to assign an appropriate density to virtual phantom for 2D diode array detector with AAA and AXB algorithms to guarantee the accuracy of patient-specific QA.
Materials and Methods1. Commissioning of Acuros XB dose calculation algorithm in water phantomFor this study, 10 VMAT plans for each of the following photon energies were selected retrospectively: 6 MV and 15 MV. All VMAT plans were generated in Eclipse TPS using Trilogy™ equipped with a millennium 120 MLC (Varian Medical Systems, Palo Alto, CA). The treatment sites were spine, head and neck (H&N), lung, brain, rectum, pelvis, cervix, and liver. VMAT plans were calculated with both AXB and AAA (version 10.0) in water phantom, and then these dose calculations were compared using dose difference criteria of 0.5%, 1.0%, 1.5%, 2.0%, and 3.0%, respectively. The percentage of agreement calculated by using dose difference criteria of n% was defined as the percentage of point which of differences between AAA and AXB were less than n%. The calculation grid of 1.5 mm for all VMAT plans was used to remove the dependency of the calculation grid.
2. Different materials of MapCHECK2 with MapPHANA 2D array dosimetry QA system, MapCHECK2 (Sun Nuclear Corporation, Melbourne, FL) has diode detectors of 0.8 mm×0.8 mm with detector spacing parallel to X and Y axes of 10.0 mm and has been used as patient-specific QA [18, 19]. The MapCHECK2 was inserted into MapPHAN phantom (Sun Nuclear Corporation, Melbourne, FL) which had a uniform mass density of 1.05 g.cm−2 for measurement. The CT images of MapCHECK2 with MapPHAN were acquired using a Brilliance CT Big Bore™ (Philips, Cleveland, OH) with slice thickness of 1.5 mm. The resolution of the CT images was 0.98 mm×0.98 mm (the size of CT voxel=1.5×0.98×0.98 mm3) with 120 kVp, 350 mAs, and collimation of 1.25 mm. The CT image was used to obtain an outline of combined MapCHECK2 with MapPAHN and then design the virtual phantom based on the outline. For evaluation of the effect of HU value or mass density on gamma index method, the virtual phantoms were artificially assigned to 4 materials of different HU values or mass densities, respectively, shown in Figure 1. Manufacturer has recommended that virtual phantom was assigned as a polystyrene (Figure 1A). In order to guarantee the accuracy of patient-specific QA, our institution found out the HU value of the virtual phantom by artificially assigning as several HU values from 200 to 500 when calculating by using AAA. The 455 HU phantom was empirically selected to show best results for patient-specific VMAT QA, named “455 HU” (Figure 1B). Jursinic et al. have recommended that MapCHECK2 and MapPHAN had different mass densities, respectively, which could improve the results of passing rate to clinically acceptable values, namely “Jusinic phantom”, as shown as Figure 1C [14]. Additionally, Polyvinyl chloride (PVC) was selected for comparison (Figure 1D). In detail, the values of HU, mass densities, and relative electron densities for 4 different assignments of the virtual phantom were listed in Table 1. All dose distributions were calculated by AAA and AXB with a calculation grid of 1.5 mm and then 2D dose distributions at the center of the virtual phantoms were exported from Eclipse TPS. Measurements were performed using MapCHECK2 inserted into MapPHAN following array calibration and absolute dose calibration.
3. Gamma evaluationPassing rates for gamma criteria of 3%/3 mm, 2%/2 mm, 2%/1 mm, and 1%/1 mm with threshold level of 10% were calculated by comparing the measured dose distribution with calculated dose distributions of 4 different materials of the virtual phantom. For all gamma index method, the global gamma evaluations were fulfilled with SNC patient software (ver. 6.1.2, Sun Nuclear Corporation, Melbourne, FL).
Results and Discussion1. Commissioning of Acuros XB dose calculation algorithm in water phantomFor validation of AXB modeling in clinic, dose distributions of VMAT plans calculated by AAA and AXB were compared for 6 MV and 15 MV. Figure 2 shows the percentages of agreement using dose difference criteria of 0.5%, 1.0%, 1.5%, 2.0%, and 3.0%, respectively. In the cases of 1.0%, 1.5%, 2.0%, and 3.0% dose difference criteria, the mean percentages of agreement for 6 MV were 97.2%±2.3%, 98.8%±1.6%, 99.4%±1.1%, and 99.9%±0.2%, respectively while those for 15 MV were 98.5%±0.85%, 99.6%±0.4%, 99.8%±0.2%, and 100.0%±0.0%, respectively, indicating that the portions of compared points which had dose difference larger than 1.5%, 2%, and 3% were greatly small for all compared points. There were minimum mean values of the percentage of agreement in the case of 0.5% dose difference criterion showing 88.1%±4.2% and 88.7%±1.5% for 6 and 15 MV, respectively. Agreement of dose distribution between AAA and AXB was slightly worse for 6 MV. Nevertheless, there were good agreements between dose distributions calculated by AAA and AXB to clinically acceptable values.
The comparisons between calculated dose distributions were not dependent on the DTA which considers the potential impact of the set-up error for measurements, thus, we only selected dose difference evaluation rather than gamma evaluation. The results showed good agreements between two calculation algorithms in the clinic. The appropriate spot sizes for modeling both AAA and AXB should be selected to fit the calculated dose distribution to measured dose distribution within acceptable tolerance level. The spot size which models the primary source (bremsstrahlung x-ray from tungsten target) affects mainly the penumbra region of the profiles for all field sizes [20]. The manufacturer provides the recommended spot sizes of 0 mm for AAA and 1 mm for AXB but a fine tuning is desirable to fit the calculations to the measurements. Fogliata et al. [20] have shown that the effect of different spot sizes of AAA and AXB on dose calculations for small field used in stereotactic body radiation treatment and then concluded that AXB was comparable to AAA following fine tuning of spot sizes was performed.
2. Different materials of the virtual phantom
Figure 3 shows the relative dose profiles in the cross-plane direction in the case of H&N for 6 MV. These were calculated by both AAA and AXB in four different materials which were polystyrene, 455 HU (our institution recommendation), Jursinic phantom (Jursinic et al. recommendation), and PVC and then normalized to prescription dose. As the HU values increased in order of polystyrene, 455 HU, and PVC, there were decreasing tendencies in relative dose profiles. The maximum values of relative dose difference in dose profiles between polystyrene and PVC were 1.77% and 1.66% for AAA and AXB, respectively, which could affect the results of gamma evaluation. AAA had more sharply peaked relative dose profiles in comparison with AXB. The reason for this was that VMAT calculation options of AAA and AXB were different under version 10 or order. As the dose distributions of VMAT were calculated in this study, calculation option “VMAT fluence resolution” which is provided in AAA version 10 was set to value “high” (fluence resolution = 0.3125 mm) to improve the calculation accuracy for small field while AXB version 10 has no this calculation option and uses resolution given by half of the value of the calculation grid size at the isocenter plane.1) Although the calculation grid for AAA and AXB was same value of 1.5 mm, fluence resolution of AAA was higher than those of AXB and then sharply peaked relative dose profiles appeared in AAA. After version 11 of AAA, the fluence resolution is determined by the used calculation grid.1)
3. Gamma evaluationMean passing rates for global gamma criteria of 3%/3 mm, 2%/2 mm, 2%/1 mm, and 1%/1 mm with threshold values of 10% were calculated as listed in Tables 2 and 3. In the case of 2%/2 mm which was recommended as a general criterion for patient-specific VMAT QA [22, 23], all mean passing rates were more than 96.0% and 97.2% for 6 MV and 15 MV, respectively, regardless of the virtual phantoms of different materials and dose calculation algorithms. There are good agreements between measured and calculated dose distributions to clinically acceptable values. The results of 3%/3 mm are always high and not sensitive to evaluate the effect of different materials of the virtual phantom. Several studies have shown that the gamma criterion of 2%/2 mm was suitable to assess the plan deliverability and accuracy of dose calculation, rather than 3%/3 mm for patient-specific VMAT QA [10, 21, 22].
For the effect of dose calculation algorithms on gamma evaluation, the results of AXB were comparable to those of AAA but had slightly decreasing tendencies, showing maximum differences of passing rates between AAA and AXB was 0.9% and 0.5% in criterion of 2%/2 mm for 6 MV and 15 MV, respectively. Those differences were not critical in the clinic. When using 455 HU recommended by our institution rather than polystyrene recommended by manufacturer, the passing rates in all criteria slightly increased for AXB as well as AAA. For the PVC and Jursinic phantom, there was no improvement of the value of passing rates. It has been demonstrated that the 455 HU was highly recommended to obtain best results of gamma evaluation but this HU value did not apply to any institution because the HU to relative electron density for a given material depends on the performance of CT of various designs, and CT scanning condition for each institution [23]. Optimal mass density or HU value of the virtual phantom should be determined for each institution.
ConclusionThis study aimed to find optimal mass density or HU values of MapCHECK2 inserted into MapPHAN to guarantee the accuracy of patient-specific QA. It has been demonstrated that 455 HU showed good agreements between measurements and calculations regardless of photon energies and dose calculation algorithms. However, this optimal material is dependent on each institution’s commissioning and could be changed.
AcknowledgementsThis study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (No. 1631200).
References1. Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310-317.
2. Park JM, Kim IH, Ye SJ, Kim K. Evaluation of treatment plans using various treatment techniques for the radiotherapy of cutaneous Kaposi’s sarcoma developed on the skin of feet. J Appl Clin Med Phys. 2014;15(6):173-187.
3. Park JM, Kim K, Chie EK, Choi CH, Ye SJ, Ha SW. RapidArc vs intensity-modulated radiation therapy for hepatocellular carcinoma: a comparative planning study. Br J Radiol. 2012;85:e323-329.
4. Park JM, Wu HG, Kim JH, Carlson JN, Kim K. The effect of MLC speed and acceleration on the plan delivery accuracy of VMAT. Br J Radiol. 2015;88:20140698.
5. Jin H, Jesseph FB, Ahmad S. A comparison study of volumetric modulated Arc therapy quality assurances using portal dosimetry and MapCHECK 2. Prog Med Phys. 2014;25(2):65-71.
6. Mattes MD, Lee JC, Elnaiem S, Guirguis A, Ikoro NC, Ashamalla H. A predictive model to guide management of the overlap region between target volume and organs at risk in prostate cancer volumetric modulated arc therapy. Radiat Oncol J. 2014;32:23-30.
7. Heo T, Ye SJ, Carlson J, Park JM. The effect of beam interruption during VMAT delivery on the delivered dose distribution. Phys Med. 2015;31:297-300.
8. Ezzell GA, et al. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: report of the IMRT subcommittee of the AAPM radiation therapy committee. Med Phys. 2003;30:2089-115.
9. Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25:656-661.
10. Fredh A, Scherman JB, Fog LS, Munck RP. Patient QA systems for rotational radiation therapy: A comparative experimental study with intentional errors. Med Phys. 2013;40:031716-1-9.
11. Daniel AL, William B, Harms SM, James AP. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25:656-661.
12. Steers JM, Fraass BA. IMRT QA: Selecting gamma criteria based on error detection sensitivity. Med Phys. 2016;43:1982-1994.
13. Ezzell GA, et al. IMRT commissioning: Multiple institution planning and dosimetry comparisons, a report from AAPM Task Group 119. Med Phys. 2009;36:5359-5373.
14. Jursinic PA, Sharma R, Reuter J. MapCHECK used for rotational IMRT measurements: Step-and-shoot, TomoTherapy, RapidArc. Med Phys. 2010;37:2837-2846.
15. Kozelka J, Robinson J, Nelms B, Zhang G, Savitskij D, Feygelman V. Optimizing the accuracy of a helical diode array dosimeter: A comprehensive calibration methodology coupled with a novel virtual inclinometer. Med Phys. 2011;38:5021-5032.
16. Borgers C. Complexity of Monte Carlo and deterministic dose-calculation methods. Phys Med Biol. 1998;43:517-528.
17. Vassiliev ON, Wareing TA, McGhee J, Failla G, Salehpour MR, Mourtada F. Validation of a new grid-based Boltzmann equation solver for dose calculation in radiotherapy with photon beams. Phys Med Biol. 2010;55:581-598.
18. Xing L, Curran B, Hill R, Holmes T, Ma L, Forster KM, Boyer AL. Dosimetric verification of a commercial inverse treatment planning system. Phys Med Biol. 1999;44:463-478.
19. Jursinic PA, Nelms BE. A 2-D diode array and analysis software for verification of intensity modulated radiation therapy delivery. Med Phys. 2003;30:870-879.
20. Fogliata A, Nicolini G, Clivio A, Vanetti E, Cozzi L. Accuracy of Acuros XB and AAA dose calculation for small fields with reference to RapidArc stereotactic treatments. Med phys. 2011;38:6228-6237.
21. Kim JI, Park SY, Kim HJ, Kim JH, Ye SJ, Park JM. The sensitivity of gamma-index method to the positioning errors of high-definition MLC in patient-specific VMAT QA for SBRT. Radiat Oncol. 2014;9:167-1-12.
Fig. 1The virtual phantoms have four difference HU values or mass densities which are (A) polystyrene, (B) 455 HU (our institution recommendation), (C) Jursinic phantom (Jursinic et al. recommendation), and (D) polyvinyl chloride (PVC). In the case of Jursinic phantom, there are two different materials assigned to MapCHECK2 and MapPHAN, respectively, while the others have one materials assigned to both MapCHECK2 and MapPHAN. 
Fig. 2The percentages of agreement between Acuros XB dose calculation algorithm (AXB) and anisotropic analytical algorithm (AAA) were calculated using dose difference criteria of 0.5%, 1.0%, 1.5%, 2.0%, and 3.0%, respectively. The dose distributions of VMAT were calculated in water. The error bars indicate the standard deviations. 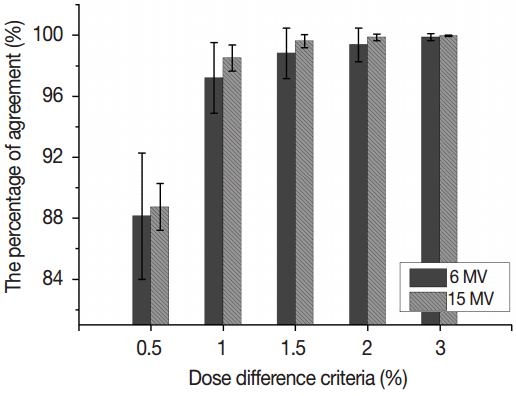
Fig. 3In the case of head and neck treated with 6 MV, the relative dose profiles for four different materials which are 455 HU (our institution recommendation), Jursinic phantom (Jursinic et al. recommendation), polystyrene, and polyvinyl chloride (PVC) are plotted as red, blue, green, and magenta, respectively. Solid and dashed lines indicate the anisotropic analytical algorithm (AAA) and Acuros XB dose calculation algorithm (AXB), respectively. 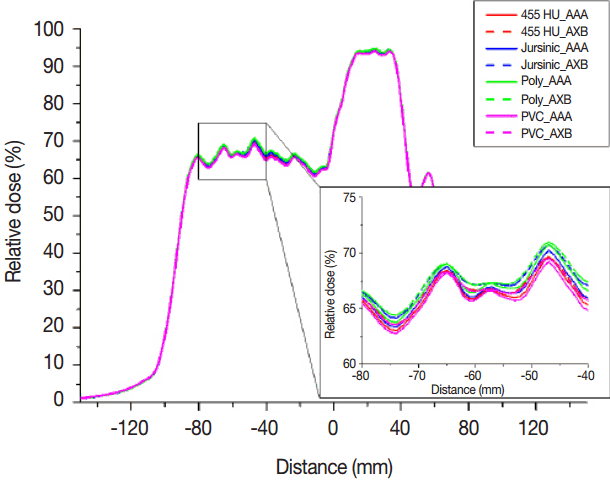
Table 1Characteristics of Four Different Combination MapCHECK2 and MapPHAN
Table 2Mean Passing Rate for Global Gamma Criteria of 3%/3 mm, 2%/2 mm, 2%/1 mm, and 1%/1 mm with Threshold Level of 10% were Calculated for 6 MV
Table 3Mean Passing Rate for Global Gamma Griteria of 3%/3 mm, 2%/2 mm, 2%/1 mm, and 1%/1 mm with Threshold Level of 10% were Calculated for 15 MV
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||