Introduction
Radiation therapy for pregnant women is limited as to the area and the timing of treatment due to concerns about fetal doses. The risks are different according to the developmental stage of the embryo or fetus. Even if the primary beam is not focused on the area of the fetus during radiation therapy, the body absorbs low doses that are difficult to predict outside the treatment area. The source of the dose outside of the radiation field is impacted by three major components: 1) treatment head leakage, 2) collimator system scattering, and 3) internal patient scattering. Advanced radiation techniques, such as intensity-modulated radiation therapy (IMRT), have higher scattered low doses than three-dimensional conformal radiation therapy (3DCRT) due to collimator scattering and head leakage [1]. Potential risks to the fetus by low doses are also unpredictable; however, a previous study reported that mental retardation and organ malformations of the fetus arise following exposure to a threshold dose of 0.5 Gy with a 50% risk of effects at 1 Gy [2]. Despite identification of such a threshold, it is important to ensure that the fetus receives as little radiation as possible. In addition, the main potential complications of radiation exposure in the growth phase of the fetus were mentioned, and in the embryonic stage, they were sensitive to 0.10 Gy of radiation or less [3]. Therefore, the radiation treatment region of a pregnant patient should be planned carefully to reduce the fetal dose to levels as low as reasonably achievable (ALARA). The concept of ALARA emphasizes the importance of minimum radiation time and maximum distance from the radiation field [4].
Tomotherapy is now widely used for treatment of head and neck (H&N) cancers due to the advantages of conformal dose distribution of the radiation field and reduction of radiation dose to critical organs [5, 6]; however, this low dose over the whole area compared to Linear Accelerator (LINAC)-based IMRT causes concern for application to pregnant women. Also, the beam-on time and monitor unit (MU) of tomotherapy are higher than those of LINAC-based 3DCRT and IMRT. Tomotherapy has not been used for pregnant women with H&N cancer, although several studies reported the results of radiotherapy for these cancers in pregnant patients [7, 8].
In this study, we evaluated the dose absorbed by the fetus during tomotherapy for patients with H&N cancer in comparison with that during 3DCRT without being shielded.
Materials and Methods
1. Selection of the patient
A 32-year-old woman who was 26 weeks pregnant with external auditory canal cancer received preoperative radiotherapy without chemotherapy. The initial clinical stage was T2N0. We defined the planning target volume (PTV) and the clinical target volume (CTV) to include the entire external auditory canal plus a 1 to 2 cm margin, resulting in a maximum diameter of 6 cm for treatment. Prophylactic neck irradiation was not performed. We prescribed the dose to the CTV and PTV as 2.0 and 1.8 Gy, respectively, in 20 fractions, and the volume of CTV and PTV were 31.2 and 84.9 mL, respectively.
2. In vitro measurement of phantom model
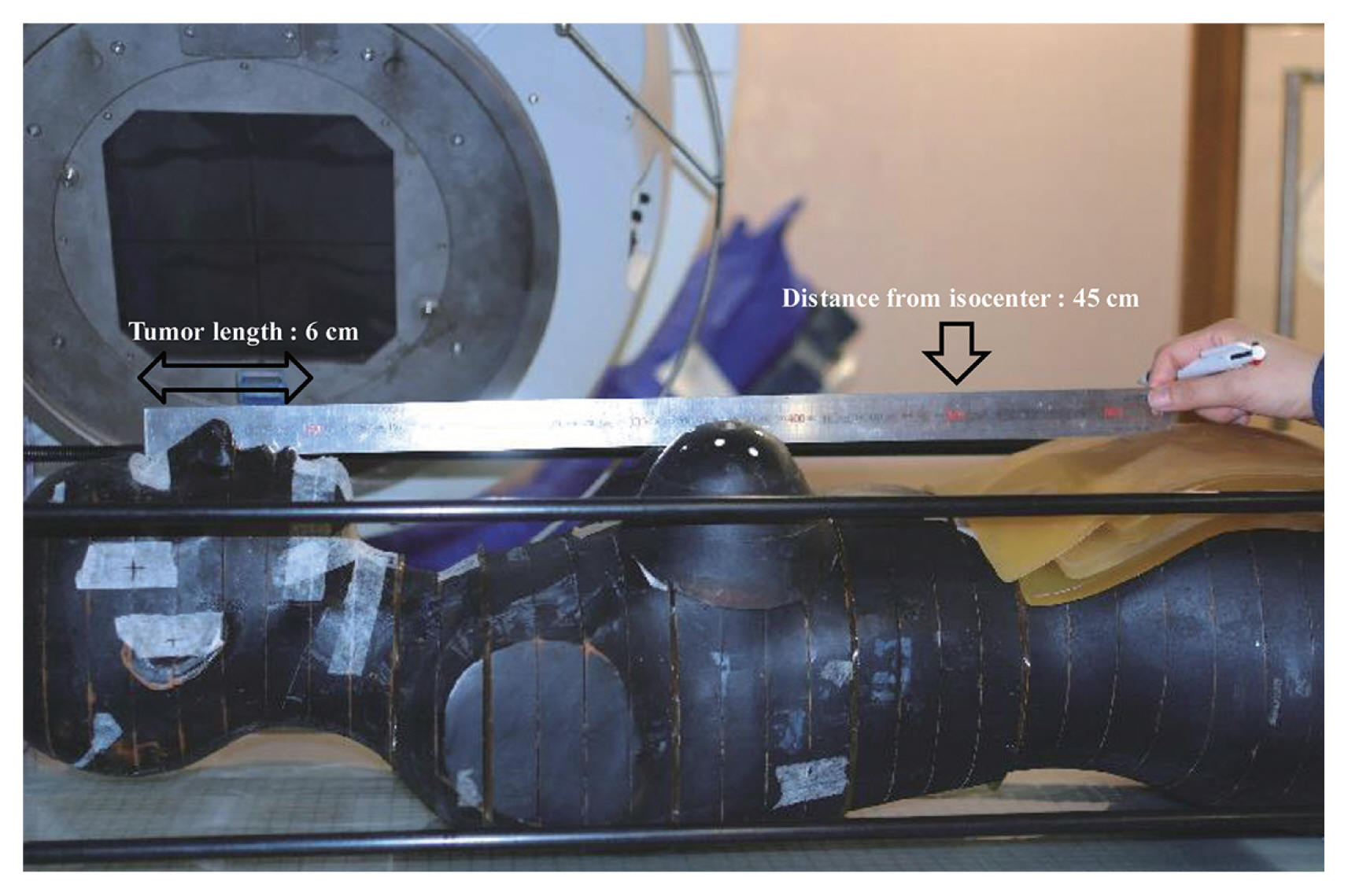
For in vitro measurements, the patient was modeled using a humanoid phantom (Alderson Research Laboratory, Long Island, NY, USA). The geometry was estimated based on the growth of the fetus and the related position of the uterine fundus. The distance from the isocenter to the fundus was 45 cm in concordance with a gestation period of 26 weeks (Figure 1) [2]. The position of the uterine fundus was expressed by applying a bolus over the phantom surface. The dose for the treatment region included the neck around the target level (i.e., 10 cm from the isocenter) and both breast areas. In the abdomen, doses on the fundus and fetal region divided in the center, anterior, and posterior were measured.
3. Tomotherapy versus 3DCRT
Tomotherapy was provided with a TomoTherapy HiArt treatment machine (Tomotherapy, Madison, WI, USA) with 6 MV and MLC of 0.625 cm widths. Tomo1 and Tomo2 plans were applied with field widths of 2.5 and 5 cm sized jaws, respectively, with a modulation factor of 2. The 3DCRT plan was carried out using a synergy linear accelerator equipped with a 1.0 cm MLC (Elekta, Synergy, Stockholm, Sweden). Three fixed beams of 6 MV were used. Tomotherapy using 2.5 and 5 cm jaws and 3DCRT were delivered with 2,144, 1,372, and 301 MU, respectively (Table 1).
We preferentially measured the same set-up doses on the 3DCRT while all leaves were closed to detect leakage and scattering, which affects the fetal dose. The same 304 MU was delivered. For comparison between tomotherapy and 3DCRT, the central dose at the fundus on each machine according to the distance from the target was measured. These fundus doses were compared between 45 cm at 26 weeks and 35 cm at 29 weeks. To compare the dose on treatment and fetus areas, we inserted groups of Optically Stimulated Luminescence Dosimeter (OSLD) between slices of the phantom and measured the dose at each position (Figure 2).
Results and Discussion
Radiation treatment for pregnant woman was performed from 26 to 29 weeks. During this period, the fundus is assumed to have a 10 cm displacement from 45 to 35 cm with a reference to the isocenter for conservative evaluation. When the out-of-field dose according to the distance of the 3DCRT was analyzed, the dose at 10 cm from the isocenter was measured as 30.5 cGy. The total dose at 25 cm from the isocenter was measured as 4.1 cGy, and the dose at the 45 cm fundus was measured at 1.7 cGy. Of the total 1.7 cGy dose received by the fundus, leakage accounted for about 1.6 cGy.
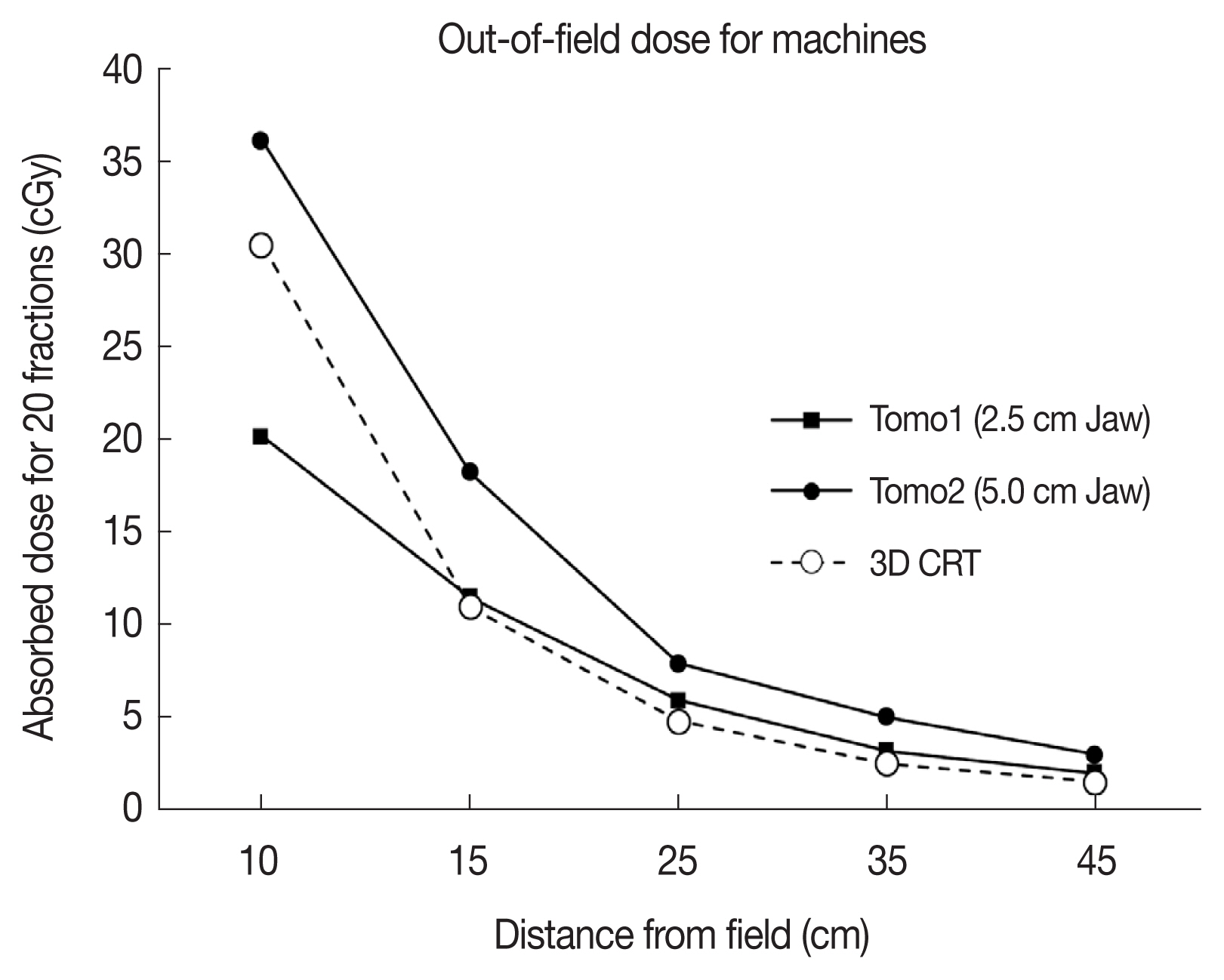
Comparison of the dose as a function of the distance from the field for each method is shown in Figure 3. The out-of-field dose of Tomo2 with a 5 cm jaw was the highest, while the dose of 3DCRT was the lowest at 15 cm from the isocenter. Doses at the uterine fundus according to different modalities were 1.7, 2.0, and 2.4 cGy for 3DCRT, Tomo1, and Tomo2, respectively. As shown in Table 2, the dose differences from 26 to 29 weeks increased to 0.7, 1.6, and 0.5 cGy for Tomo1, Tomo2, and 3DCRT, respectively. The Tomo2 plan showed an increased rate of 30% more than the other modalities. Table 3 and Figure 4 show the measured doses from the patient and the fetus region according to each modality. For the prescribed dose of 40 Gy, the Tomo2 plan delivered the highest dose of 36.13 Gy, while Tomo1 delivered the lowest dose of 20.18 Gy to the neck. All modalities rapidly decreased to about 20% of the prescribed dose in the breast area, which was 25 cm away from the isocenter. Most of the fetus region received less than 5% of the prescribed dose, although the Tomo2 plan delivered a higher dose to the fetus region than the other modalities.
Among various cancers, H&N cancer requires particular caution regarding the peripheral radiation dose, as this type of cancer requires both high doses and longer treatment periods than other cancers. We evaluated a 32-year-old pregnant woman with external auditory cancer and recommended tomotherapy as an appropriate treatment modality. Using a humanoid phantom, our study confirmed that both tomotherapy and 3DCRT have a low impact on the fetus, which received less than 5% of the prescribed patient dose. Tomotherapy delivers more peripheral radiation to the fetal region due to the 5-fold longer beam-on time compared with 3DCRT. When comparing tomotherapy regimens, however, the effect on fetal dose appears to be more influenced by collimator scattering based on field size and leakage dose by the MLC than by the beam-on times. In accord with these results, another study reported that the peripheral dose was not prominently increased by beam-on time but by the collimator and leakage scattering by a larger field size [1]. Our result also showed that the Tomo1 with a 2.5 cm jaw had higher MU than the Tomo2 with a 5.0 cm jaw, but fetus dose was higher in Tomo2. The 3DCRT measured dose also confirmed that leakage irradiation is the major component, accounting for about 85% of the 1.64 cGy delivered to the fundus. It remains important, however, to analyze the effect of MU on peripheral dose. Because the radiation treatment plans represent different MU values, the peripheral irradiation dose and machine scattering are related to the MU [9]. Previous studies emphasized that the integral dose, which is affected by the out-of-field doses, is related to the MU, indicating that head leakage and collimator scatter increase as MU increases [10, 11].
From the target field, the radiation dose received by the fetus region was estimated to be about 5% of the prescribed dose of 40 Gy. The dose decreased rapidly to about 20 cm or more from the isocenter (Table 3 and Figure 4). The peripheral dose was attenuated as the distance from the target increased, and the majority of the dose from the target results from leakage and scattering, as reported by Matthew B et al. [12]. These investigators showed that applying appropriate shielding for these peripheral doses can reduce the radiation doses to less than 50% of those without shielding on the H&N cancer patient.
The radiation doses received by the fetus area were uniform in this study; however, the fetus position changes during radiation treatment as well as during gestation. Thus, it is important to consider the fetus position during the treatment period. Table 2 shows the dose effect on the fetus position; more doses of radiation were delivered to the same position after 3 additional weeks of gestation. Between 26 and 29 weeks, doses of Tomo1 (2.5 cm jaw), Tomo2 (5 cm jaw), and 3DCRT increased 0.7 cGy (35%), 1.6 cGy (69.6%), and 0.5 cGy (31.3%), respectively. Dose increases by tomotherapy was proportional to jaw size. The dose at out-field of irradiation in the Tomo2 plan was about 30% higher than that by 3DCRT despite superior dose conformity. Moreover, the 5 cm jaw plan delivered 20% of the off-field dose compared to the 2.5 cm jaw plan, demonstrating the significant contribution of head scatter irradiation in tomotherapy. As a drawback, however, we need to measure the head leakage of tomotherapy although our preliminary study confirmed tomotherapy have different leakage dose according to the machine even in equal conditions.
This study did not evaluate post-shielding doses at different points of the fetus, but the results suggest appropriate shielding condition for each patient as the measured peripheral area doses were determined without shielding. In addition, image dose by scan range in the tomotherapy based on the image of megavoltage computed tomography (MVCT) is also important to the low dose regions. The further study is needed to measure in vivo dosimetry with actual patients using shielding conditions and acquiring the MVCT image to confirm the protection from radiation treatment.
Conclusion
In conclusion, we evaluated the radiation dose received by the fetus during preoperative radiotherapy for H&N cancer. From the results of present study, we recommend tomotherapy with a 2.5 cm jaw for radiation treatment of pregnant patients with H&N cancer. With 3DCRT, a major component of radiation dose is leakage irradiation, which is proportional to the MU. In tomotherapy, the fundus dose is highly dependent on head scattering, supporting a lower off-field dose with a smaller jaw size. Although tomotherapy is superior in normal tissue sparing, the fundus dose was about 20 to 30% higher than that with 3DCRT. Off-field irradiation is always dependent on dose, dose-volume, field size, and MU, and overall, the evaluation of risk of tomotherapy should be individually performed for each patient.