Determination of 226Ra in TENORM Sample Considering Radon Leakage Correction
Article information
Abstract
Background
Phosphogypsum is material produced as a byproduct in fertilizer industry and is generally used for building materials. This material may contain enhanced radium-226 (226Ra) activity concentration compared to its natural concentration that may lead to indoor radon accumulation. Therefore, an accurate measurement method is proposed in this study to determine 226Ra activity concentration in phosphogypsum sample, considering the potential radon leakage from the sample container.
Materials and Methods
The International Atomic Energy Agency (IAEA) phosphogypsum reference material was used as a sample in this study. High-purity germanium (HPGe) gamma spectrometry was used to measure the activity concentration of the 226Ra decay products, i.e., 214Bi and 214Pb. Marinelli beakers sealed with three different sealing methods were used as sample containers. Due to the potential leakage of radon from the Marinelli beaker (MB), correction to the activity concentration resulted in gamma spectrometry is needed. Therefore, the leaked fraction of radon escaped from the sample container was calculated and added to the gamma spectrometry measured values.
Results and Discussion
Total activity concentration of 226Ra was determined by summing up the activity concentration from gamma spectrometry measurement and calculated concentration from radon leakage correction method. The results obtained from 214Bi peak were 723.4±4.0 Bq·kg−1 in MB1 and 719.2±3.5 Bq·kg−1 in MB2 that showed about 5% discrepancy compared to the certified activity. Besides, results obtained from 214Pb peak were 741.9±3.6 Bq·kg−1 in MB1 and 740.1±3.4 Bq·kg−1 in MB2 that showed about 2% difference compared to the certified activity measurement of 226Ra concentration activity.
Conclusion
The results show that radon leakage correction was calculated with insignificant discrepancy to the certified values and provided improvement to the gamma spectrometry. Therefore, measuring 226Ra activity concentration in TENORM (technologically enhanced naturally occurring radioactive material) sample using radon leakage correction can be concluded as a convenient and accurate method that can be easily conducted with simple calculation.
Introduction
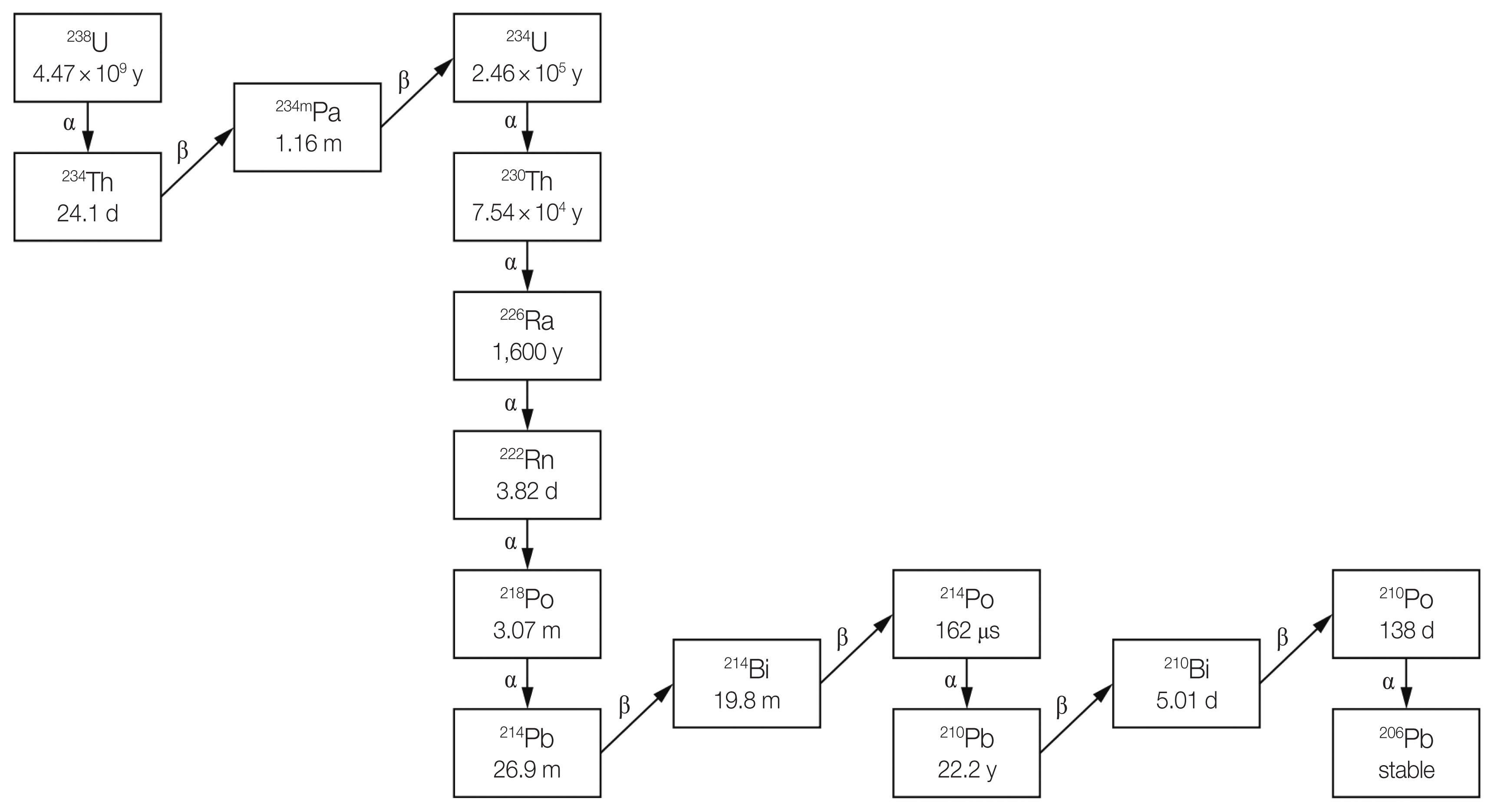
Naturally occurring radioactive material, generally known as NORM is material containing natural radionuclides such as uranium (U), thorium (Th), and actinium (Ac) series, and also 40K and few cosmogenic radionuclides. Radium-226 (226Ra), one of the decay products of the uranium series, is an important radionuclide from radiological risk point of view. This nuclide has half-life of 1,600 years and decays by emitting alpha and gamma radiation to produce radon-222 (222Rn), the only gaseous radionuclide of the decay series as shown in Fig. 1 [1]. 222Rn can easily enter the human body through respiration system and affect lung with high energy alpha radiation as it decays. Hence, the World Health Organization recognized 222Rn as a secondary cause of lung cancer after smoking [2].
The activity concentration of 226Ra in soil from natural environment in Korea varies from 6.31 to 135 Bq·kg−1 and averaged as 39.4 Bq·kg−1 [3]. While in the United States, the activity concentration of 226Ra in soil are in the range of 8–160 Bq·kg−1 with an average of 40 Bq·kg−1 and uranium concentration in phosphate ores found to be in the range of 20–300 ppm or about 0.26–3.7 Bq·g−1 and thorium occurs at essentially background levels, between 1–5 ppm (or about 0.0037–0.022 Bq·g−1) [4, 5]. However, with some specific situations, the activity concentration can be enhanced by human activities such as mining and industrial activity including fertilizer production. The material with enhanced activity concentration is known as TENORM (technologically enhanced naturally occurring radioactive material). Phosphogypsum generated in fertilizer production is classified as TENORM by the United States Environmental Protection Agency [5].
Phosphogypsum is generally used for building materials such as phosphate board and cement, which may cause accumulation of indoor radon. Recently, the Korea Land & Housing Corporation established a guideline for the reduction of radioactive materials in building material [6]. According to the guideline, activity concentration of 226Ra was set for ≤130 Bq·kg−1, as a standard of proper building material. Although phosphogypsum is no longer used in Korea as building material, some buildings using phosphogypsum constructed in the past still exist. Thus, on a regulatory perspective, the measurement method for 226Ra activity concentration still needs to be developed.
Many researches on the measurement of the 226Ra activity concentration as the source of 222Rn exhalation from the phosphogypsum have been conducted. Direct method measures 186.2 keV photon energy peak of 226Ra. However, 185.7 keV peak from 235U interrupts the peak of 226Ra, this interval between 185.7 keV and 186.2 keV is so close that even HPGe detector with good resolution cannot identify both nuclides. Thus, additional alpha spectrometry is needed to subtract the portion of 235U. Otherwise, an indirect method measures 295.0 keV of 214Pb and 609.3 keV of 214Bi peaks. This method needs to suppose that 226Ra and its daughters to be in equilibrium. Thus, to determine 226Ra precisely by this method, it takes about 28 days to achieve secular equilibrium [7].
To avoid these disadvantages, a research was performed to study the correction factor estimated from the natural abundance ratio of 238U and 235U. However, the correction factor cannot be implemented on measuring 226Ra concentration in phosphogypsum TENORM because the secular equilibrium between 226Ra and 238U has been interrupted [8]. Unlike natural materials, phosphogypsum as a by-product of phosphatic fertilizer is produced by chemical process that separates uranium in phosphoric acid from radium in phosphogypsum. As a result, phosphogypsum contains higher activity concentration of 226Ra compared to its 238U parent nuclide [9].
Furthermore, another consideration on 226Ra measurement in indirect gamma spectrometry is 222Rn leakage from the sample container. Since 222Rn is an inert gas, it can easily escape from the sample container even with a tiny crack of gap between container and its lid. A research related to radon tightness of sample container in radium activity measurement of soil sample has also be done. This research using indirect method by high-purity germanium (HPGe) detector and closed-loop radon measurement method (RAD7) to determine the tightness of sample container using three different sealing methods. Marinelli beaker was used in this research, and its release fractions of the three sealing methods were obtained from the proportion of radon gas that released from the sample container to total free radon concentration potentially released to the air from soil in Marinelli beaker. This method, however, requires specific equipment to seal the container tightly [10].
Therefore, this research evokes a measurement method of 226Ra activity concentration of phosphogypsum by gamma-spectrometry using HPGe detector where leak-correction factor is applied for compensating the radium underestimation caused by the released 222Rn especially on the radon leaked container. Results are compared with, first, the certified value of phosphogypsum as a reference material, and second, 226Ra activity from the phosphogypsum in properly sealed sample container.
Materials and Methods
1. Phosphogypsum Sample and Sample Container Sealing Methods
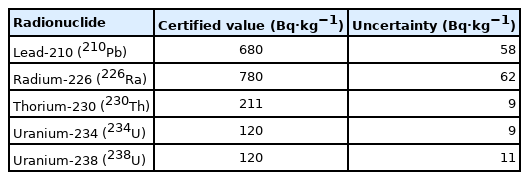
The International Atomic Energy Agency (IAEA) phosphogypsum reference material was used as a sample in this study. This sample is certified for 210Pb, 226Ra, 230Th, 234U, and 238U nuclides as shown in Table 1. The activity concentration of the sample was certified on January 1, 2008, then activity concentration of 226Ra once the measurement was conducted (July 1, 2019) was calculated as 776.1±62 Bq·kg−1 using its half-life. In this study, an indirect method is used to analyze 226Ra, therefore radioactivity concentration between 226Ra and daughter nuclides is considered. After 21 days of measurement, daughter nuclides’ activity reaches 97.7% of the decay-calculated 226Ra concentration (776.1±62 Bq·kg−1), which is 758.8±61 Bq·kg−1.
The physical form of the sample was powder typed that was put into 450 mL Marinelli beaker (MB) container that is widely used for gamma spectrometry with HPGe detector. The degree of tightness of the three different sealing methods (MB1, MB2, MB3) that has been studied previously were used as given in Table 2. Radon leakage from MB1 type sample container was 25%, MB2 type was 14% and MB3 type was not determined because released radon from MB3 type sample container was at the background level.
2. Measurement Method
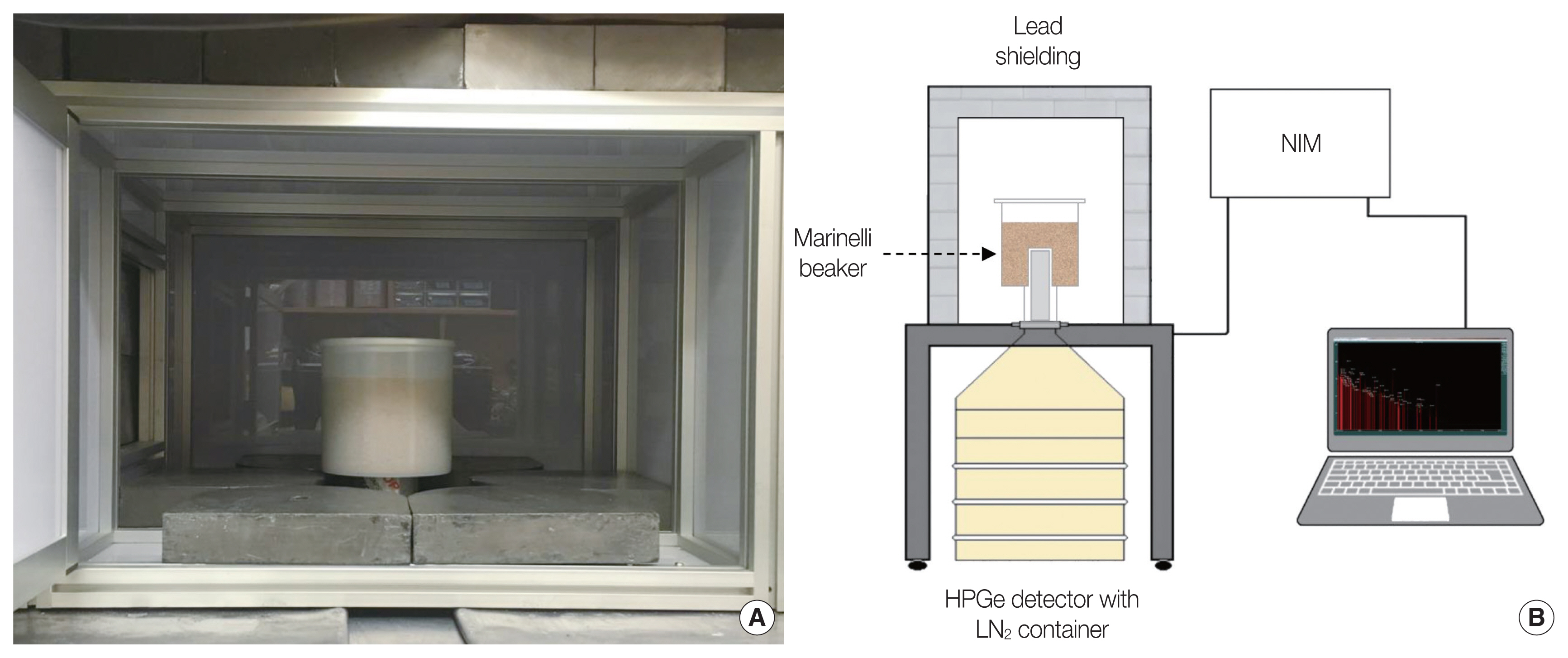
Gamma spectrometry using HPGe detector (GEM15P4; Ortec, Oak Ridge, TN, USA) was conducted to measure 226Ra activity concentration in phosphogypsum sample. The sample was measured with HPGe detector in lead chamber which is made of 5 cm-thick lead brick to shield background gamma rays as shown in Fig. 2. Prior to sample measurement, the detector efficiency calibration was performed using a reference material certified by the Korea Research Institute of Standards and Science (KRISS). The reference material contained multiple nuclides with gamma energy peaks range from 59.54 keV to 1,836 keV. Thereafter, the sample was sealed with the following order of MB1, MB2, and MB3 and each sealed sample was measured for 21 days continuously, data were taken for every 84,600 seconds. Phosphogypsum sample was purged after 21-day measurement to remove the 222Rn gas that might exist in the sample. Each gamma spectrum was taken to analyze peaks of 226Ra, 214Pb, and 214Bi after subtraction of background peaks. Background peaks were measured for 84,600 seconds with empty sample container.
Results and Discussion
1. Gamma Spectrometry Results
The HPGe detector measures gamma rays as nuclides decay in the sample. It provides gamma-ray energy spectrum so that nuclides can be identified by its energy peaks. Two daughter nuclides of 226Ra (214Pb and 214Bi) emit gamma rays with high yields, i.e., 295.0 keV with 18.42% yield from 214Pb and 609.3 keV with 45.49% yield from 214Bi. Therefore, to obtain 226Ra activity concentration, 214Pb and 214Bi activity concentrations in phosphogypsum in three different sealing methods were measured by gamma spectrometry, and the results are depicted in Fig. 3A and 3B. Some data points were missing in MB3 curve because the gamma spectrometry system lost its power for several days during the 21-day measurement.
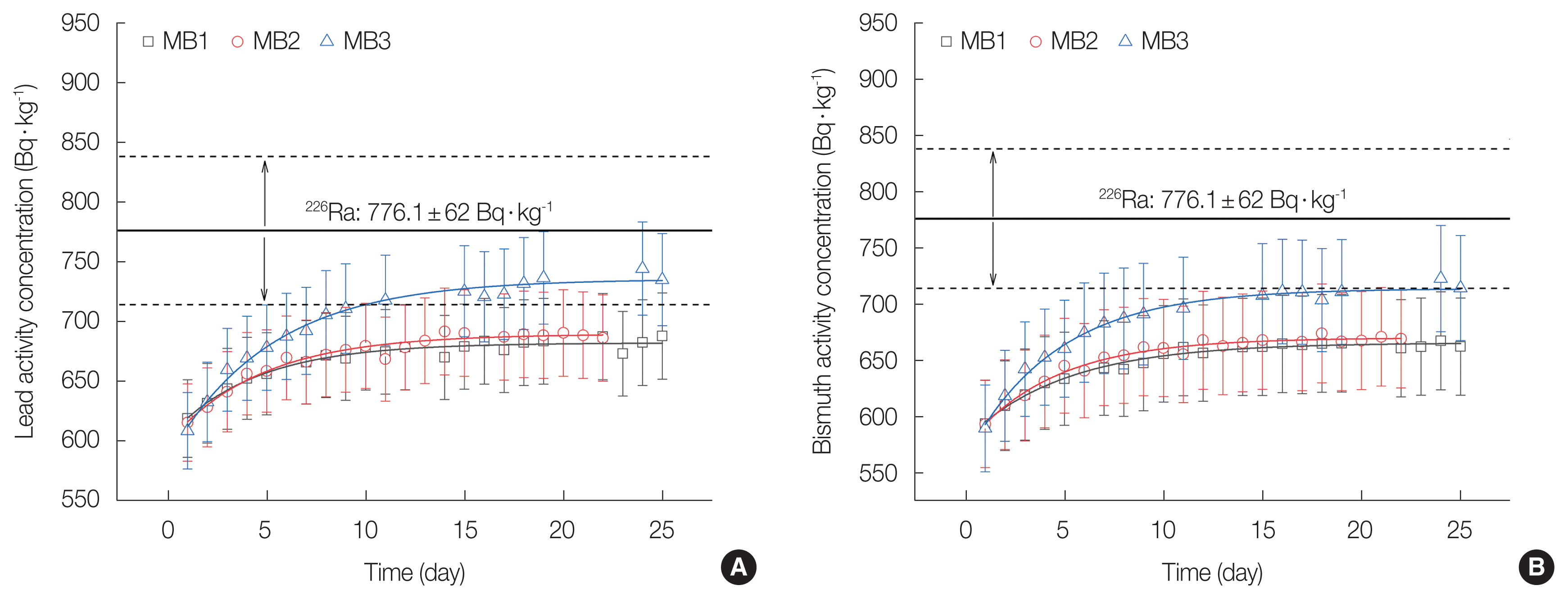
High-purity germanium measured result of activity concentration build-up for MB1, MB2, and MB3 sealing method for (A) 214Pb and (B) 214Bi in phosphogypsum sample. MB, Marinelli beaker.
In both Fig. 3A and 3B, daily measured values were depicted as points and that increased towards the line of certified value (776.1±62 Bq·kg−1) from IAEA reference material that has considered it’s decay during the period from the reference date and measured date. If there was no leak from the sample container, the measured value of the activity concentration of 214Bi and 214Pb should reach 97.7% of the certified value (758.8±61 Bq·kg−1) by radioactive equilibrium between radium and its daughter nuclides after 21 days from the beginning of the masurement. However, for both 214Bi and 214Pb, there were discrepancies between the measured value of 21-day and certified value, which indicates the leakage from the sample container existing in all three sealing methods.
Activity concentrations of 214Bi and 214Pb in Fig. 3A and 3B resulted during the measurement period show that concentrations of both nuclides were built-up and saturated in the sample container. Daily measured activity concentrations were fitted with Origin 2018 program to analyze the saturated value and compared to the IAEA certified value. Saturated values of 214Bi were 665.7±1.3 Bq·kg−1, 670.2±1.4 Bq·kg−1, and 713.9±2.0 Bq·kg−1, respectively for MB1, MB2, and MB3, while saturated values of 214Pb were 681.9±1.6 Bq·kg−1, 689.3± 2.0 Bq·kg−1, and 735.4±2.7 Bq·kg−1, respectively on MB1, MB2, and MB3. The result of both 214Bi and 214Pb showed that as the sealing of the container gets tighter, the radon leakage reduced. Especially on the MB3 sealing method, saturated value of 214Bi had 8% difference from certified value of 226Ra of phosphogypsum sample that is within the uncertainty bound. In the same way, 214Pb had 5% difference from certified value that is also within the uncertainty bound while other sealing methods showed more than 10% differences that is outside the uncertainty bound.
The result above indicates that MB3 is the proper sealing method that can be used to measure 226Ra activity concentration in phosphogypsum sample with an indirect method of HPGe gamma spectrometry. The measurement result of 214Bi was slightly less than 214Pb and that cause larger difference to compare with the certified value. It is due to the 214Bi result needs to be corrected, related to its true coincident summing effect to prevent underestimation of its activity concentration [11]. Thus, in this study, 214Pb was used to analyze 226Ra concentration.
The measurement results also show that 226Ra activity concentration is smaller than certified value in MB1 and MB2 due to 222Rn leakage. 222Rn is an inert gas that is easy to escape through the tiny space of sample container without reaction with other materials. The ratio of radon leakage or radon leakage fraction, as shown in Table 2, is the physical characteristics of container that is not affected by sample activity. Thus, radon leakage fraction can be used to correct 222Rn leakage of the phosphogypsum sample in this study. To compensate the leaked portion of measured value, some assumptions are used and explained in leakage correction method.
2. Leakage Correction Method
Taking the advantage of secular equilibrium properties between 226Ra and 222Rn, in this study, 226Ra activity concentration was obtained by adding up the non-leaked and leaked concentration of radon from the sample container. The non-leaked radon was represented by its daughter (214Pb, 214Bi) saturated activity concentration measured by HPGe, further defined as measured activity concentration, while the leaked radon was estimated using equations below. The leaked radon is further defined as estimated activity concentration. The estimation of leaked radon is based on an assumption that leaked 222Rn gas establishes secular radioactive equilibrium with 214Pb or 214Bi in a space with a certain volume. If N2 is the number of radon daughter nuclide atoms, then the rate of change for radon daughter nuclides can be expressed with generation term and decay term as shown in the following differential equation:
where N1 is the number of 222Rn atoms, λ1 and λ2 are decay coefficient of 222Rn and 214Pb, respectively, and k is radon leakage fraction of Marinelli beaker as given in Table 2 that represent a leak proportion of radon gas from the sample container. For MB1, MB2, and MB3, k value was 0.25, 0.14, and about 0 which showed background level that can be neglected.
By solving Equation (1)N2(t) can be derived as follows:
where N1(0) indicates an initial value of the number of 222Rn atoms, which is the first 86,400 seconds measured value of radon daughter activity concentration in this study. Because the decay constant is 19.9 minutes for 214Bi and 26.8 minutes for 214Pb then it can be assumed that 222Rn and its daughter nuclides are in equilibrium. Thus, A2(t), activity of radon daughter nuclides, can be obtained by multiplying λ2 to Equation (2).
The radon leakage fraction (k) in Equation (1) to (3) indicates that if the sample container is sealed perfectly and no radon leaked (k=0), then the estimated activity concentration (A2) will be zero, then the total activity concentration will be equal to the measured activity, which shows an ideal container sealing condition.
3. Correction Result
Results of 226Ra activity concentration using leak correction method are presented in Table 3 with used parameters. Estimated activity concentration was obtained from the Equation (1) to (3) that represents leaked portion of 222Rn gas from sample container and measured activity concentration was obtained from indirect gamma spectrometry that represents saturated 222Rn gas in sample container. Besides, leakage-corrected 226Ra activity concentration that is the summation of estimated and measured activity concentration of 226Ra is also shown in Table 3. Also, leakage-corrected 226Ra activity concentration was compared with certified 226Ra activity concentration of IAEA reference material and the difference between them was calculated.
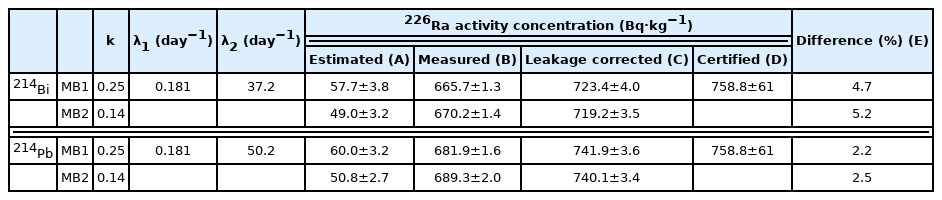
Results of 226Ra Activity Concentration Using Leakage Correction Method and Gamma Spectrometry for 214Bi and 214Pb
Results demonstrate that MB2 is tighter sealing method than MB1 as estimated results of MB1 were higher than MB2 and measured results were smaller in both 214Bi and 214Pb commonly. However, results of 214Bi total activity concentration showed small discrepancy from certified values, because of coincident summing effect was not considered in this study, while results of 214Pb total activity concentration showed almost correspondence with certified value with about 2%.
Thus, it can be concluded that a proper method for determining 226Ra activity concentration is introduced in this study when the indirect method of gamma spectrometry using 214Pb energy peaks with leak correction method is used. This method only requires a Marinelli beaker and an initial measurement value for calculation and the measurement value of 21-day. Nonetheless, this result is specific for the Marinelli beaker as sample container, while for other sample containers, a new radon leakage fraction is required. For further study, coincidence summing effect of 214Bi will be considered for radon leak correction method.
Conclusion
In this study, the activity concentration of 226Ra in the phosphogypsum, TENORM from fertilizer industry, sample was measured using HPGe gamma spectrometry. Determining 226Ra in phosphogypsum has many challenges such as interruption of 186.2 keV 235U gamma peak to its peak in direct measurement, and interruption of radioactive secular equilibrium between 238U and 226Ra by radon leakage from the sample container. Therefore, in this study, a proper measurement method for determining 226Ra using indirect gamma spectrometry combined with radon leakage correction method for different sealing method of sample container was studied.
Measurement results of indirect method using 226Ra daughter nuclides (214Bi and 214Pb) showed continuous build-up and saturation of 222Rn in the phosphogypsum sample. Activity concentration in MB3 sealing method showed very close value to the IAEA certified value while the results of MB1 and MB2 cases had discrepancy from the certified value in both 214Bi and 214Pb. The discrepancy was caused by the leaked radon, therefore leak correction was added to the indirect gamma spectrometry to obtain the 226Ra activity concentration.
Total activity concentration of 226Ra obtained from measured and calculated results of leak correction method. Results using 214Bi peaks were 723.4±4.0 Bq·kg−1 in MB1 and 719.2± 3.5 Bq·kg−1 in MB2 that showed discrepancy of about 5% compared to the certified activity. Besides, results using 214Pb peaks were 741.9±3.6 Bq·kg−1 in MB1 and 740.1±3.4 Bq·kg−1 in MB2 that showed about 2% differences compared to the certified activity.
Therefore, measuring 226Ra activity concentration in TENORM sample using radon leakage correction can be concluded as a convenient and accurate method that can be easily conducted with simple calculation. For further research, true coincidence summing effect can be corrected with Monte Carlo N-Particle (MCNP) simulation to acquire accurate results of 214Bi peaks.
Acknowledgements
This research was supported by Kyungpook National University Development Project Research Fund (2018).
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contribution
Conceptualization: Lim S. Data curation: Lim S, Syam N. Formal analysis: Lim S, Syam N. Funding acquisition: Lee SH. Methodology: Lim S, Syam N. Project administration: Syam N, Lee SH. Visualization: Lim S. Writing - original draft: Lim S. Writing - review & editing: Maeng S, Lee SH. Resources: Syam N. Supervision: Syam N, Maeng S. Validation: Lim S, Syam N, Maeng S.