Effects of Vitamin E Derivative TMG on the Radiation Protector and Tumor Growth during Radiotherapy
Article information
Abstract
Background
The purpose of this study is to evaluate the immunosuppressive and antioxidant effects of a novel radioprotective agent using the vitamin E derivative 2-(alpha-D-glucopyranosyl)methyl-2,5,7,8-tetramethylchroman-6-ol (TMG) and its effect on tumors, and to study its usefulness.
Materials and Methods
In this study, C57BL/6NCrSlc mice were divided into four groups (control, TMG, radiation therapy [RT], and RT+TMG), using 10 mice in each group. In the TMG and 2 Gy+TMG groups, 500 mg/kg TMG was administered. Two groups (2 Gy and 2 Gy+ TMG) among RT and RT+TMG groups were irradiated with 2 Gy in a single fraction, while the other two groups (6 Gy and 6 Gy+TMG) were irradiated locally with 6 Gy in three fractions.
Results and Discussion
TMG positively affected CD4+ and CD8+ T lymphocytes. Tumor volumes and growth inhibition rates were compared. In order to evaluate how TMG administration affected tumor growth, Ehrlich cancer cells were injected into the thigh of mice, and the tumor volume and growth suppression rate were compared. Not only RT but also TMG alone inhibited tumor growth. If RT conducted to the mice with TMG, TMG could increase the number of leukocytes, primarily that of lymphocytes. TMG also inhibited tumor growth in addition to RT. Tumor growth was significantly inhibited in the 6 Gy+TMG group.
Conclusion
In conclusion, TMG exerted an immunopotentiating effect mainly by increasing the white blood cell numbers including that of lymphocytes. In addition to RT, TMG also inhibited tumor growth. Therefore, TMG is considered to be a useful radioprotective agent in radiotherapy without tumor growth induction.
Introduction
For decades, several various technical improvements have been implemented for cancer treatment. In the field of radiation therapy (RT), intensity-modulated RT, image-guided RT, and proton therapy have evolved and have been frequently used in clinical practice [1]. All these physical-based techniques are developed to deliver higher doses to the tumor, and lower doses to the normal organs. However, it is still inevitable to irradiate a certain amount of normal tissue and to avoid radiation-induced complications.
Therefore, several biologically acting radioprotectors have been investigated [2]. Although numerous materials have been studied, no compound could be widely accepted and used so far in clinical practice due to toxicity and concerns related to influencing tumor growth itself [2, 3].
Recently, significant interest has emerged in immune therapy [4, 5]. The central concept of traditional radiobiology was the DNA double-strand break-based cytotoxic effect of radiation on tumor cells, leading the damaged cells into apoptosis, necrosis, or autophagy. However, a newly defined form of cell death, immunogenic cell death is an emerging concept and received relevant interest these days [5]. The representative effect of immunogenic cell death is the abscopal effect. It is a term describing radiotherapy-induced tumor regression in targets away from a targeted lesion. However, this is not observed in lymphocyte-deficient nude mice [5, 6]. Many studies have reported that host immunity and T cells are essential for the immunogenic cell death [4–6].
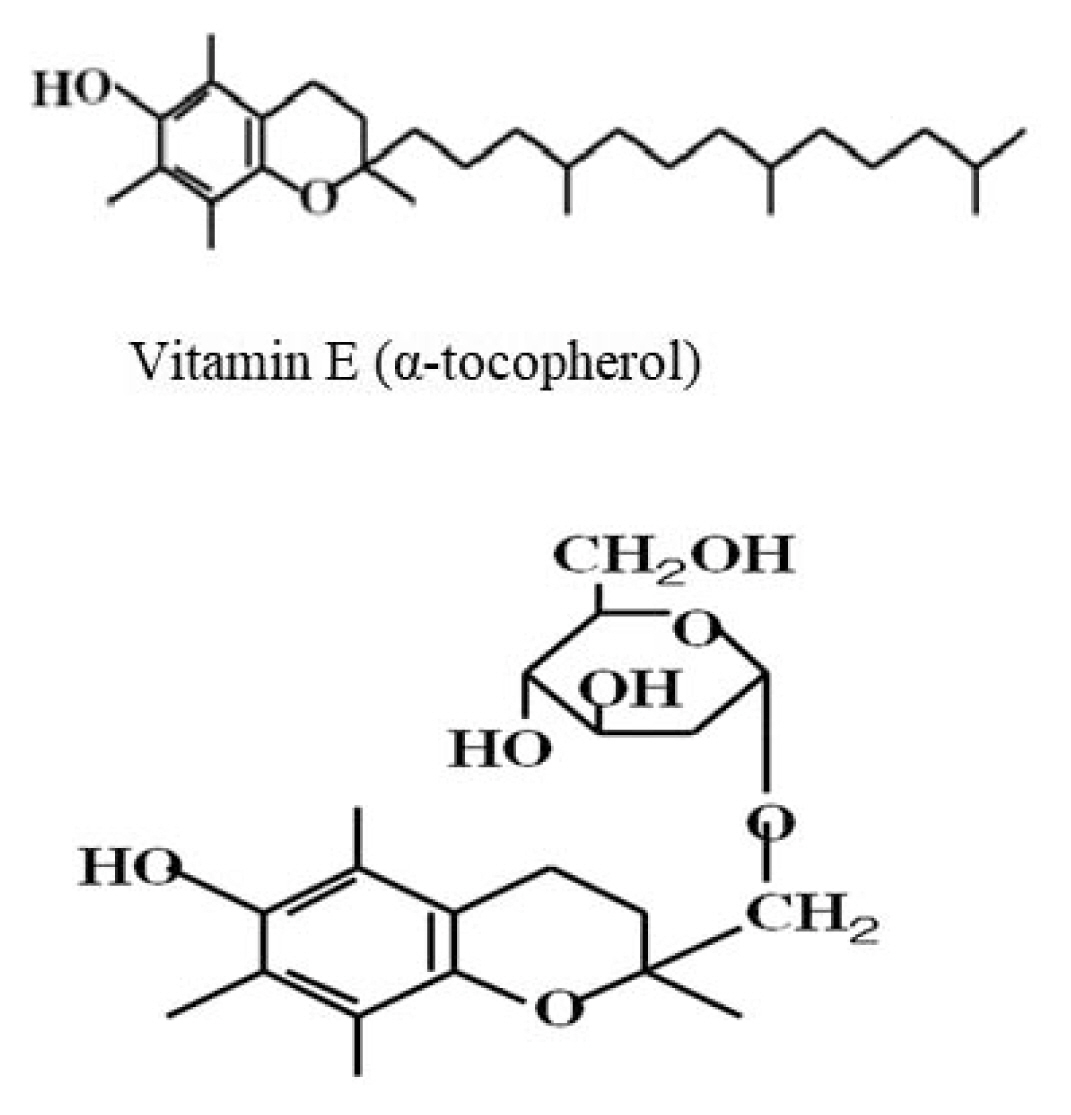
Vitamin E (VE) is also called α-tocopherol [7, 8]. 2-(alpha-D-glucopyranosyl)methyl-2,5,7,8-tetramethylchroman-6-ol (TMG) is a lipid-soluble vitamin, a genetic description for all tocopherols and tocotrienols [7], and is abundantly present in wheat germ oil, sunflower, and safflower oils. Currently, several studies have reported VE to exert powerful antioxidant properties by scavenging free radicals which are critical in radiation-induced cell damage [9]. However, how VE affects the immune system and tumor growth remains elusive. Therefore, we performed an experiment to explore the aforementioned elusive questions in conjunction with RT using a mouse model.
Materials and Methods
1. Animals and Maintenance
Three-week old C57BL/6NCrSlc mice (body weight 8–13 g) were purchased from Japan SLC (Shizuoka, Japan) and were housed in a temperature-controlled room maintained at 22±3 °C with relative humidity of 60% on a 12-hour light/dark cycle. They were given experimental diets (CE-2; CLEA, Tokyo, Japan) and water ad libitum. All mice were acclimated to laboratory conditions for 1 week before the experiments.
2. Radiation Delivery
Radiation was delivered using the MG226/4.5 system (Philips, Best, The Netherlands), an X-ray generator developed for animal irradiation. Under the conditions of tube voltage 200 kV, dose rate of 0.35 Gy/min, and applying an additional filter composed of 0.1 mm Cu and 1 mm Al, radiation was delivered as local thigh or whole-body irradiations. The prescribed dose was 6 Gy in three fractions every other day and 2 Gy in a single fraction for local thigh and whole-body irradiations, respectively.
For local irradiation, only the right thigh of the mouse was exposed to the round irradiation field, and other sites were completely blocked by a lead shield to diminish the scattered radiation. For whole body irradiation, the mice were confined to a small plastic dish that was rotated at a constant speed.
3. Exp 1: Changes in White Blood Cell Numbers
For this experiment, four groups (control, TMG, RT, and RT+TMG groups) of Institute of Cancer Research mice were used, with 10 animals in each group. TMG was administered by diet in the TMG and RT+TMG groups at a level of 500 mg/kg until the end of the experiment (day 36). In the control and RT groups, purified water was added. Radiation was delivered locally on the right lateral thigh 6 Gy in three fractions every other day in the RT and RT+TMG groups starting on day 14. A 10 μL of peripheral blood was obtained from the caudal vein with a capillary tube, and the number of leucocytes and lymphocytes was counted using an automated hematology analyzer (Celltac α+ MEK-6318; Nihonkouden Co. Ltd., Tokyo, Japan). The count of total leukocytes, lymphocytes, granulocytes, and monocytes was recorded seven times on days 14, 16, 20, 22, 24, 28, and 36.
4. Exp 2: Changes in T Lymphocytes
The experimental groups were also classified as control, TMG, RT, and RT+TMG groups as in Exp 1. TMG was also administered in the TMG and RT+TMG group as in Exp 1 until the end of the experiment (day 38). In the control and RT groups, purified water was administered. Whole-body radiation (2 Gy) was delivered on day 28. Whole blood was collected from the mouse hearts under anesthesia and mixed with heparin on days 27, 35, and 38. Flow cytometry reagents for lymphocyte subset measurements were added to the lymphocyte suspension in phosphate-buffered saline (PBS), and the mixture was stained for immunofluorescent imaging for approximately 30 minutes at 4 °C in a dark room. After the reaction, the solution was rinsed three times with PBS, and the CD3+, CD4+, and CD8+ subsets were analyzed with a FACSCaliber flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). To analyze T lymphocyte subsets, we used a multicolor flow cytometry system (Santa Cruz Biotechnology Inc., Dallas, TX, USA), and CD3+, CD4+, and CD8+ T lymphocytes in the peripheral blood were counted in three-color flow cytometry using antiCD3-PE-Cy5.5, antiCD4-FITC, and antiCD8-PE dyes.
5. Exp 3: Effect on Tumor Growth
The same four groups were used to test the effect on tumor growth. Ehrlich carcinoma cells (1×106 cells) were injected into the right lateral thigh of the mice. TMG was administered in the diet in the TMG and RT+TMG groups at a level of 500 mg/kg until the end of the experiment (day 36). Radiation was delivered locally on the right lateral thigh at 6 Gy in three fractions in the RT and RT+TMG groups starting on day 14. From day 7, the shortest and longest tumor diameters were measured every other day using the Vernier caliper, and the tumor volume was calculated as follows:
On day 36, the tumor was removed to measure its weight, and the tumor growth inhibition rate was calculated as follows:
where WC and WT are the mean tumor weight of the control group and the mean tumor weight of the treated groups (RT, TMG, and VE+RT groups), respectively.
6. Exp 4: Effect on the TNF-α Level
To test how TMG affected the tumor necrosis factor-α (TNF-α) level, four groups (control, TMG, 6 Gy, and TMG+ 6 Gy groups) were compared, comprising five C3H mice in each group. TMG was intraperitoneally administered in the TMG group for 21 days at the same level. The TNF-α level measurement was carried out by enzyme-linked immunosorbent assay (ELISA) using a mouse TNF-α ELISA kit (Pierce Biotechnology Inc., Waltham, MA, USA). The 50 μL of sample and the standard were distributed to each well and incubated at room temperature (20–25 °C) for 120 minutes after covering with a plate cover. Then, 50 mL of biotinylated antibody reagent was added to each well, and the samples were incubated at room temperature for 2 hours. After washing five times with the wash buffer, 100 mL of horseradish peroxidase solution was added and the wells were covered with a plate cover. After incubation at room temperature for 30 minutes, washing was carried out five times with a wash buffer. A 100 μL of tetramethylbenzidine solution was added and incubation was carried out for more than 30 minutes. The incubation time was determined according to the extent of blue color development. The incubation was terminated by adding 100 mL of stop buffer into the samples. Absorbance at 450 nm was measured using a Labsystems Multiskan MS-UV (Dainippon Pharmaceutical Co. Ltd., Osaka, Japan) within 30 minutes after the stop buffer supplementation and the amount of TNF-α was estimated based on the absorbance using a standard calibration curve.
7. TMG
VE is a fat-soluble vitamin and is also called tocopherol. Tocopherol is a methylated derivative of tocopherol. The cyclic part in the structure is commonly called a chroman, and there are four types, α, β, γ, and δ, depending on the position and presence of the methyl group attached to the chroman. In humans, D-α-tocopherol is used, possessing the strongest activity and potentially acting mainly as an antioxidant. It is abundant in wheat germ, sunflower, and safflower oils. TMG is an abbreviation for 2-(α-D-glucopyranosyl)methyl-2,5,7,8-tetramethylchromann-6-ol, which is a water soluble artificial VE obtained by adding glucose to VE (Fig. 1). In the past, it was developed through joint research between the Gu Laboratory of Junshin Gakuen University and the Kagiya Laboratory of Kyoto University.
Results and Discussion
1. Exp 1: Changes in white blood cell numbers
Figs. 2, 3 present the changes in the blood leukocytes. The leukocyte number increased significantly in the TMG group compared to the control (p<0.01) after 24 hours (Fig. 2A). In both irradiated groups (RT and RT+TMG groups), the number of leukocytes decreased until 3 hours and recovered from 12 hours, from days 7 to 30. However, the magnitude of recovery was higher in the RT+TMG group compared to the RT group (p<0.05) (Fig. 2B). Figs. 2, 3 show that the leukocyte-related changes were affected largely by the changes in the lymphocytes than the monocytes rather than that of the granulocytes. The granulocyte numbers did differ significantly between the control and TMG groups. Granulocyte recovery after the RT did not differ between the RT and RT+TMG groups either. The granulocyte number was higher in the RT group on from days 7 to 30.
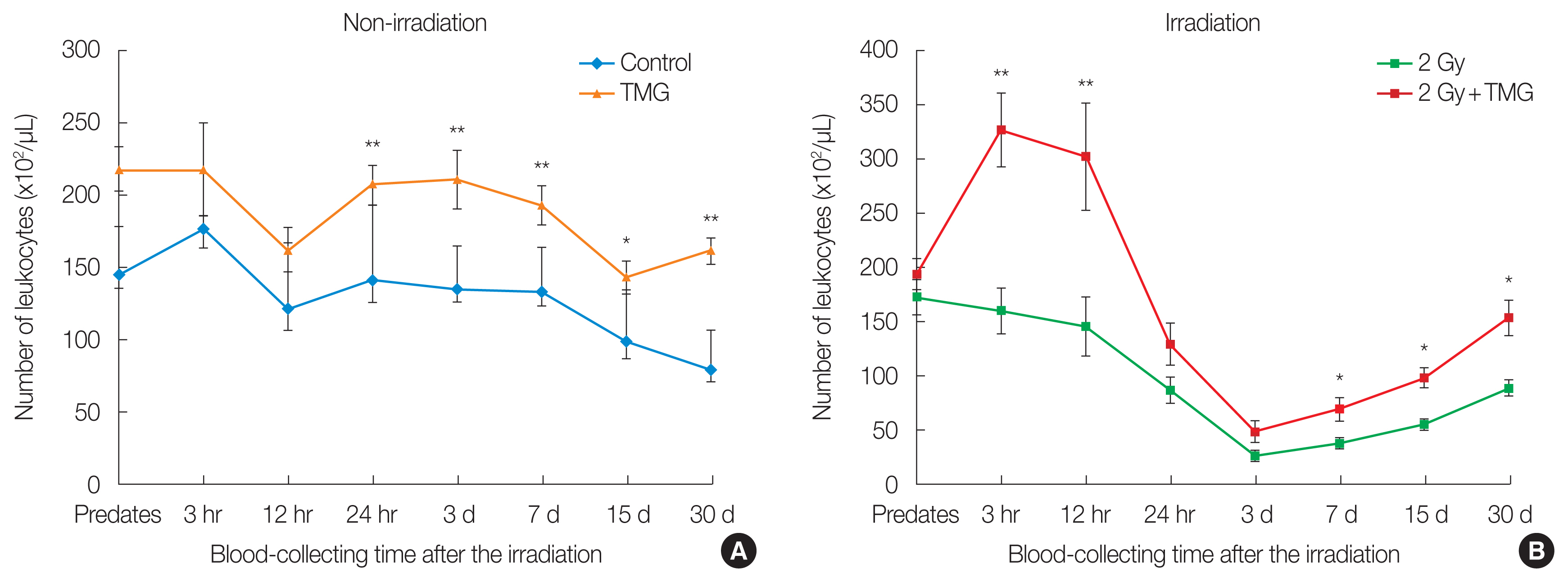
Effect of TMG on blood leukocytes counts in irradiated mice. Leukocytes are the total number of white blood cells. Statistically significantly different from the control group (*p<0.05, **p<0.01). TMG, 2-(alpha-D-glucopyranosyl)methyl-2,5,7,8-tetramethylchroman-6-ol.
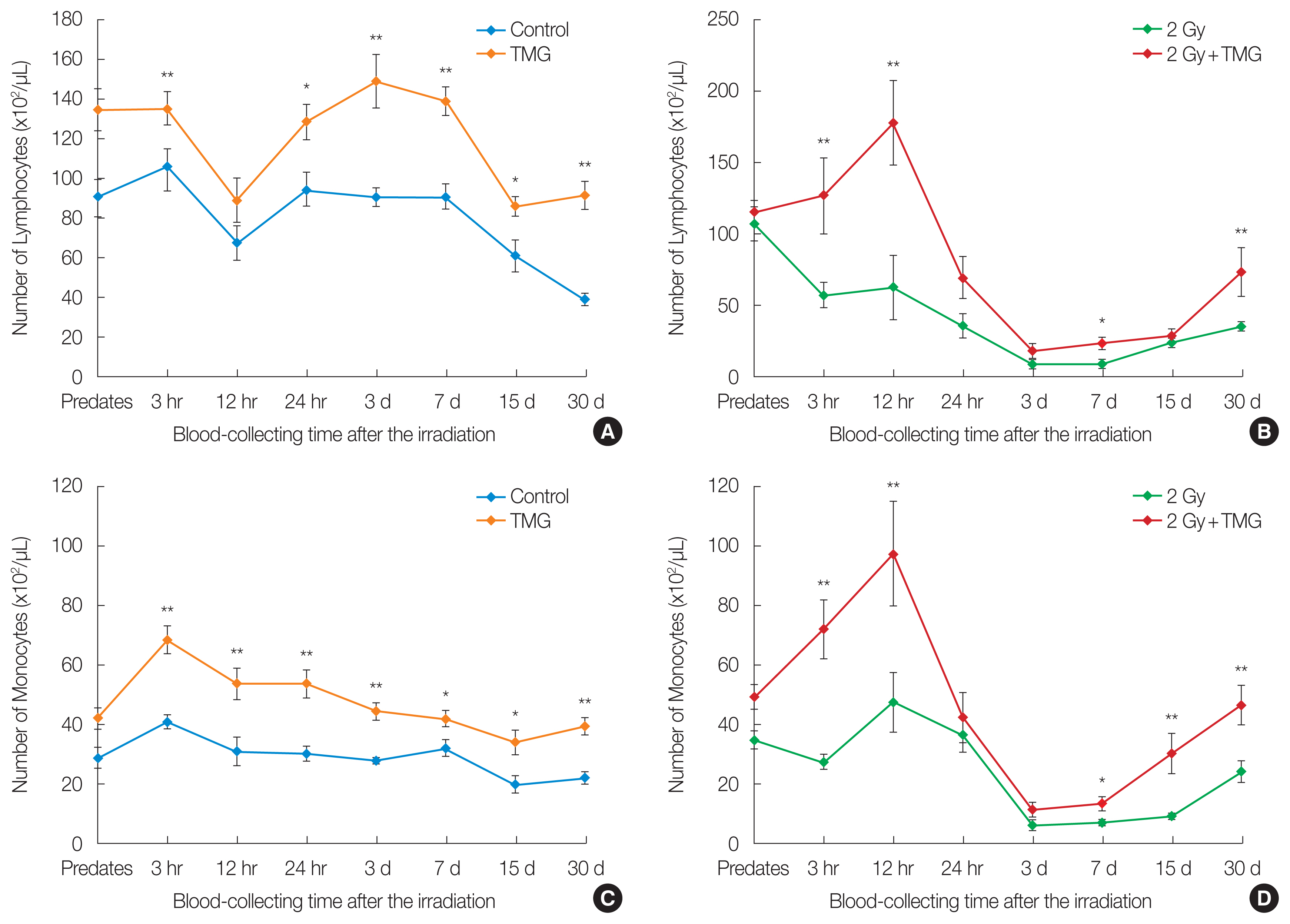
Effect of TMG on blood lymphocyte and monocytes counts in irradiated group of 2 Gy in mice: (A, C) non-irradiation and (B, D) irradiation. Leukocytes are the total number of white blood cells. Lymphocyte and monocytes counts are parts of white blood cells. Statistically significantly different from the control group (*p<0.05, **p<0.01). TMG, 2-(alpha-D-glucopyranosyl)methyl-2,5,7,8-tetramethylchroman-6-ol.
2. Exp 2: Changes in T Lymphocytes
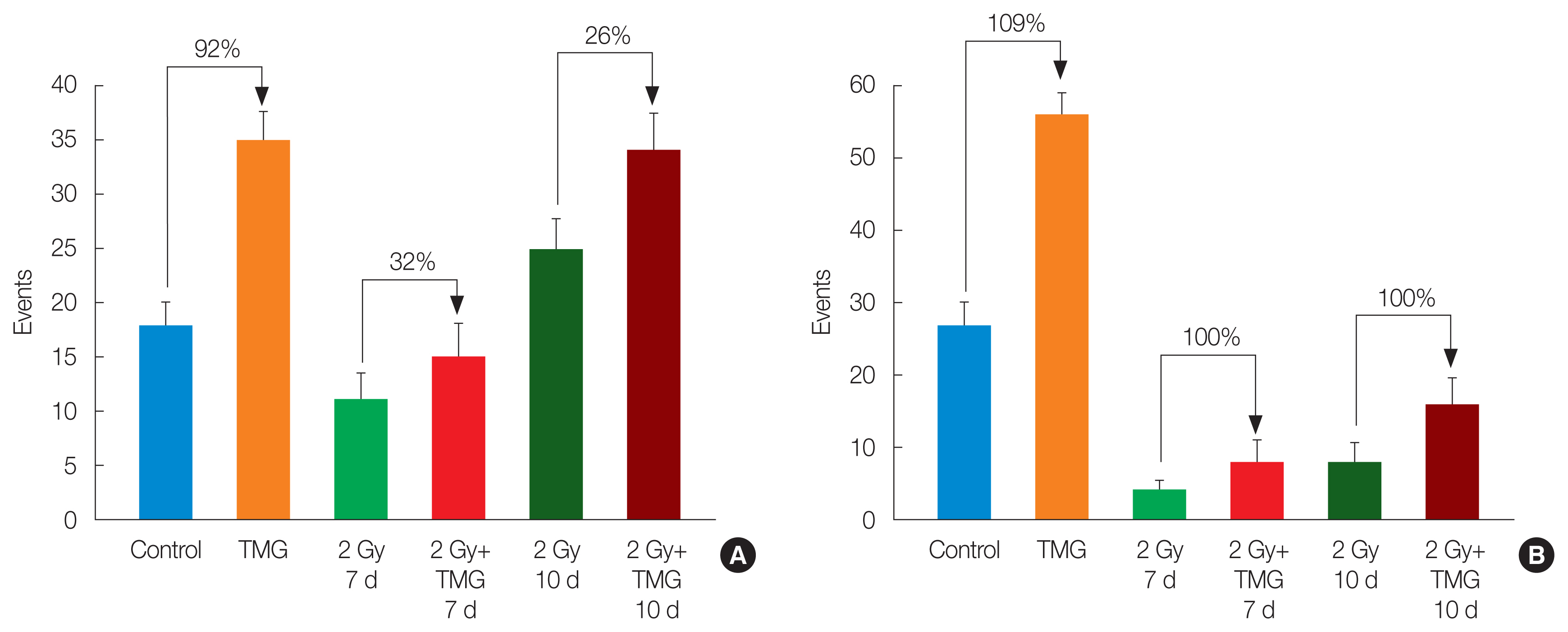
Since the protective TMG effect was greatest for the lymphocytes, we analyzed which type of lymphocytes increased. Fig. 4 presents the flow cytogram and the CD4+ and CD8+ cell dot plot.
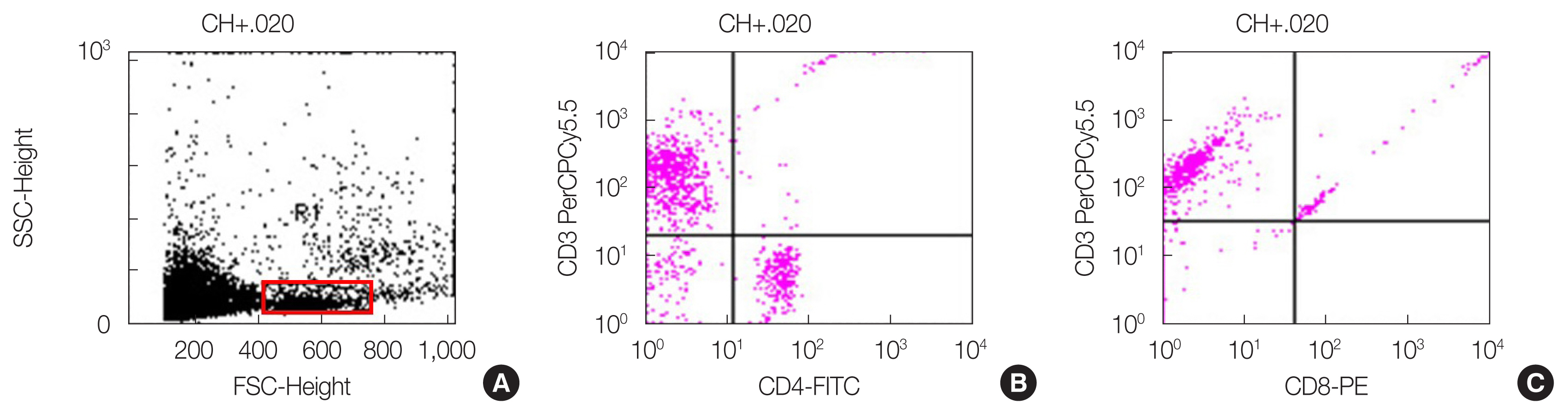
(A) Flow cytogram, the flow cytometric results of (B) CD4+ cells and (C) CD8+ cells used by five mice. SSC, side scatter; FSC, forward scatter; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
The CD4+ cells increased by 92% in the TMG group compared to the control. The VE also increased the CD4+ cells in the irradiated groups by 32% and 26% on days 35 (1 week after RT) and 38 (10 days after radiation), respectively (Fig. 5A). The CD8+ cells also showed similar results. The CD8+ cell number in the TMG group was higher than that in the control. In the irradiated groups, the CD8+ cell number in the TMG+RT group was double of that in the RT group on days 35 and 38 (Fig. 5B).
3. Exp 3: Effect on Tumor Growth
Table 1 and Fig. 6A show the tumor volume in the control and TMG groups. Even without any cytotoxic treatment, the tumor volume was smaller in the TMG group after day 23. The difference became more significant with time. Table 1 and Fig. 6B present the tumor volume in the irradiated groups, being also smaller in the TMG+RT group than in the RT group after day 25 and the difference increased with time.
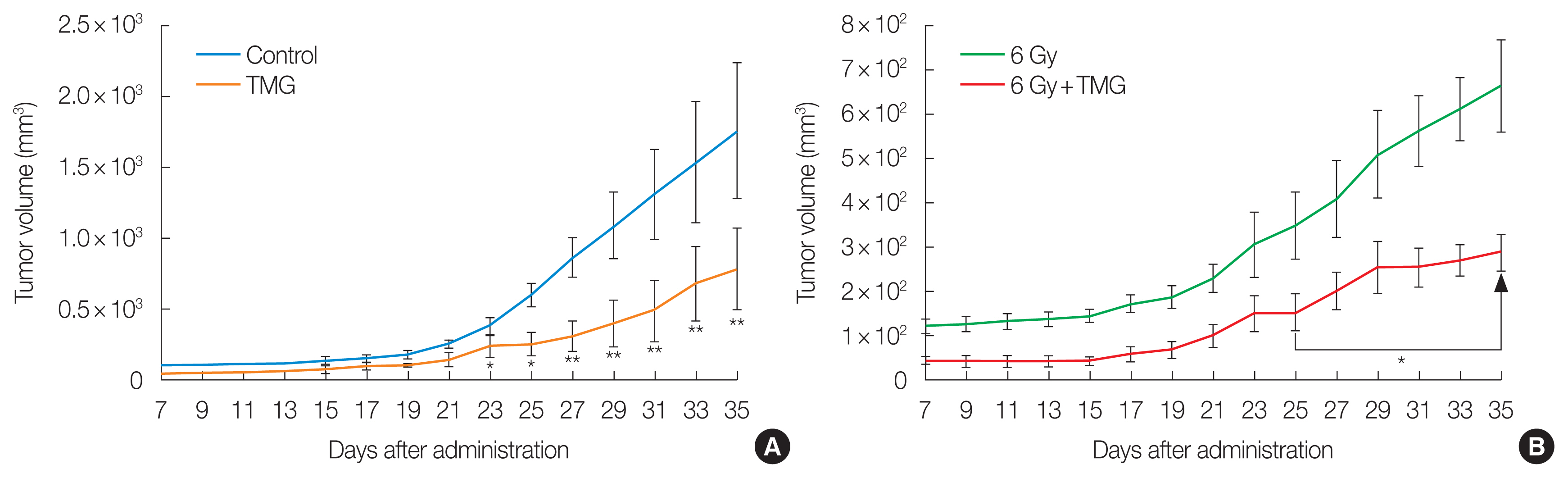
Effect of vitamin E derivative TMG on tumor growth in (A) unirradiated groups and (B) irradiated groups inoculated with Ehrlich carcinoma cells. Statistically significantly different from the control group (*p<0.05, **p<0.01). TMG, 2-(alpha-D-glucopyranosyl)methyl-2,5,7,8-tetramethylchroman-6-ol.
Table 1 highlights the tumor weight and growth inhibition rate in each group. The average tumor weight was 1.28 and 0.29 g in the control, RT, and TMG groups, respectively. When TMG and RT were both administered, the average tumor weight was only 0.01 g. The tumor growth inhibition rates were 95.8% and 98.8% in RT and TMG+RT groups, respectively, displaying statistically significant differences from the control (p<0.05 and p<0.01, respectively).
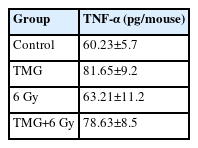
4. Exp 4: Effect on the TNF-α Level
The TNF-α level acquired from the optical density is shown in Table 2. The TNF-α level was higher in the TMG administration group both in the un-irradiated groups (control vs. TMG groups) and irradiated groups (RT vs. TMG+RT groups). The TNF-α level was less than 20% in the TMG group compared to the control (p<0.01). TNF-α was also increased as RT was administered, and it was higher in the TMG+RT group compared to RT alone group (p<0.05).
5. Discussion
Hematologic toxicity is the most common complication that can be observed in patients with cancer treated by chemotherapy or RT [10]. In particular, when leukocytopenia develops the patient could suffer from severe infection, potentially leading to death. However, from the aspect of immune cancer therapy, hematologic toxicity is more than a complication. Several studies have reported that the immune system plays an important role in cancer prevention and control. Especially, recently published studies highlighted the role of lymphocytes in tumor control [6, 11]. These studies indicate that patients with intense lymphocytic infiltrate around the tumor exhibited improved tumor control and survival. In addition, patients with comorbidities that caused immunosuppression or decreased lymphocytes level displayed poor survival outcomes. High neutrophil-lymphocyte ratios also correlated with poor prognosis in several cancer types with higher metastasis rates and recurrence [12, 13]. Although the underlying mechanism remains unclear, it could be associated with increased neutrophil-dependent inflammation and reduced lymphocyte-mediated tumor response [13, 14]. Therefore, protecting the lymphocytes from cytotoxic therapies is very important not only for reducing treatment complications but also for increasing the probability of cancer treatment.
In our study, TMG increased the number of leukocytes overall mainly due to the lymphocyte rather than the granulocyte increase. Not only CD4+ but also CD8+ cells increased by the VE administration. This effect could also be observed in irradiated mice. Lymphocytes are the most radiosensitive cells in the body, and in vitro studies suggest that the dose required to kill 50% of the (D50) lymphocyte population is approximately 1 Gy, while the D90 is approximately 2 Gy [15]. In our study, the recovery of overall leukocytes and lymphocytes occurred faster in the RT+TMG group compared to the RT group. Several studies reported that for proper host immunity activation, both CD8+ cytotoxic and CD4+ helper T cells are essential to show an immune reaction to the tumor.
Although the underlying mechanism of how VE increases lymphocyte numbers remains unclear, one explanation could be the antioxidant effect of VE. Several studies described the antioxidant effect of TMG [8, 9]. Pekmezci [8] reported reduced chemotherapeutic and RT toxicities by reducing free radicals. Another explanation is the direct effect of VE on the immune system.
All materials that could act as radioprotectors raise concerns in tumor control since they might also protect the tumor from RT damage, leading to reduced local control [16]. Amifostine, the only Food and Drug Administration-approved radioprotective drug, also exhibits a potentially hazardous effect on tumor control, although this topic remains controversial [3, 17]. However, in our study, TMG showed no deteriorative effect on tumor control. VE rather displayed a tumor cell killing effect by itself (control vs. TMG groups) as well as an additive effect on tumor control to RT [18–22].
The exact TMG effector mechanism on tumor control remains unknown. However, based on the increased TNF-α level and lymphocyte/monocyte numbers in the TMG group, we think TMG could increase immune reaction against the tumor. TNF-α is a cytokine involved in acute systemic inflammation, produced mainly by activated macrophages, as well as by CD4+ lymphocytes and NK cells [18]. It can trigger apoptosis in various tumors and also regulate the immune cells [18, 19].
TMG is an effective antioxidant with no significant acute toxicity when administered orally at a dose of 1 or 2 g. As TMG is water soluble, it displays global antioxidant and radiation protection benefits. If effective, TMG could penetrate and become a global protector. Different blood barrier amifostine indicates adequate single-dose safety [23–25].
Approximately 1 and 3 weeks in the control and TMG groups, respectively, on days when the size of the swelling exceeded 10 times. It took approximately 3 weeks in the illuminated group and about 4 weeks in the TMG+illuminated group. In addition, a sensitizer was used when performing the radiation treatment to provide therapeutic effects.
The results of this study could be further improved. However, so far, side effects have often been a problem with protective agents and have been remained as a future task.
Conclusion
Based on our results, we conclude that TMG could increase the leukocyte numbers, mainly that of the lymphocytes, thereby preventing hematologic toxicity in patients treated with RT. In addition to RT, TMG also helps to inhibit tumor growth. Tumor growth inhibition could be due to the effect of increased lymphocytes and TNF-α levels.
In addition to the immune aspects involved in the TMG administration-mediated helper and cytotoxic T cell enhancement, it causes radical scavenging of OH-radicals and a reduced blood cell count.
This suggests that TMG exhibits an immunopotentiating and a radioprotective effect on immune cells. In addition, we clarified that Ehrlich cancer cells showed an antitumor effect and the radioprotective effect on the tumor was negligible. Based on these pieces of evidence, if a new protective agent could be developed from this compound in the future, treatment could be performed without lowering patient immunity, leading to noninvasive and highly effective radiotherapy. In addition, since it might be effective in reducing immune cells, which is a side effect of chemotherapy, it should be advanced to studies in combination with anticancer agents.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Ethical Statement
All animal care and experimental protocols were approved by the Research Ethics Committee of Suzuka University of Medical Science (Ref No. 2019/207/625/37).
Author Contribution
Conceptualization: Gu YH, Matsumoto R. Data Curation: Matsumoto R, Yamashita T. Writing – original draft: Gu YH, Matsumoto R. Writing – review & editing: Gu YH.