A Simple and Effective Purification Method for Removal of U(VI) from Soil-Flushing Effluent Using Precipitation: Distillation Process for Clearance
Article information
Abstract
Background
The purpose of this study is to purify uranium (U[VI])-contaminated soil-flushing effluent using the precipitation–distillation process for clearance. Precipitation and distillation are commonly used techniques for water treatment. We propose using a combination of these methods for the simple and effective removal of U(VI) ions from soil-flushing effluents. In addition, the U concentration (Bq/g) of solid waste generated in the proposed treatment process was analyzed to confirm whether it satisfies the clearance level.
Materials and Methods
Uranium-contaminated soil was decontaminated by soil-flushing using 0.5 M sulfuric acid. The soil-flushing effluent was treated with sodium hydroxide powder to precipitate U(VI) ions, and the remaining U(VI) ions were removed by phosphate addition. The effluent from which U(VI) ions were removed was distilled for reuse as a soil-flushing eluent.
Results and Discussion
The purification method using the precipitation–distillation process proposed in this study effectively removes U(VI) ions from U-contaminated soil-flushing effluent. In addition, most of the solid waste generated in the purification process satisfied the clearance level.
Conclusion
The proposed purification process is considered to have potential as a soil-flushing effluent treatment method to reduce the amount of radioactive waste generated.
Introduction
Because nuclear energy has a high energy density, it is a more reliable source of electricity than renewable energies such as solar, hydro, and wind [1, 2]. In addition, nuclear energy is a useful energy source in terms of slowing climate change by reducing carbon dioxide (CO2) emissions [3–5]. However, the uranium (U[VI]) used to produce nuclear energy poses potential environmental hazards because of its radio- and bio-toxicity [6–8].
In South Korea, a large amount of soil contaminated with U(VI) was generated during the decommissioning of the U(VI)-conversion plant operated by the Korea Atomic Energy Research Institute. This soil waste is being stored in a radioactive waste storage facility awaiting management and disposal [9–11]. Therefore, it is necessary to develop a technology for treating the soil contaminated with U(VI) to reduce the amount of stored radioactive waste and economically dispose of it. Soil decontamination can be performed by various methods such as soil-washing, soil-flushing, electrokinetic extraction, and phytoremediation [12–17]. Among these decontamination methods, soil-washing and soil-flushing are commonly used to remove metals from soil [18]. However, compared with a number of studies on soil-washing, fewer studies on soil-flushing have been reported. Soil-washing is a batch-type treatment method in which the soil and washing solution are thoroughly mixed, whereas soil-flushing is a column-type treatment method in which the solution is percolated through the soil. The soil-flushing has the advantage of consuming less eluent and having lower operating costs than soil-washing [19, 20]. Even when U(VI) is removed from the soil, the wastewater from wet soil decontamination is a secondary waste and requires remediation. Metal ions, including U(VI), can be removed from wastewater by various methods such as chemical precipitation, ion exchange, distillation, and electrochemical treatment [21, 22]. Among these methods, the chemical precipitation method can be applied to the waste liquid treatment process simply and practically [23, 24].
Our research team has reported the results of a study on how to decontaminate U(VI)-contaminated soil to the clearance level (1 Bq/g) by soil-washing [25]. In addition, U(VI) ions were removed from the soil-washing effluent by neutralization–precipitation using sodium hydroxide (NaOH) powder [25]. In the present study, U(VI)-contaminated soil was decontaminated by a soil-flushing method to reduce the amount of effluent. The soil-flushing effluent was purified using precipitation and distillation methods. The U(VI) concentration in the soil decontaminated by soil-flushing and in the solid waste generated during the precipitation–distillation process was investigated, and the possibility of clearance of these solid wastes was confirmed.
Materials and Methods
1. Materials
The U-contaminated soil used in the present study was prepared by dry separation of U-contaminated soil stored in the waste storage facility of the Korea Atomic Energy Research Institute; the separation was performed using a <2 mm sieve (#10; Chunggye, Seoul, Korea). The initial concentration of U(VI) in the U-contaminated soil was 23.2 Bq/g. Sulfuric acid (H2SO4, 98%), NaOH (98%), and potassium phosphate monobasic (KH2PO4, extra pure) were purchased from Duksan Chemicals.
2. Preparation of U-Contaminated Soil-Flushing Effluent
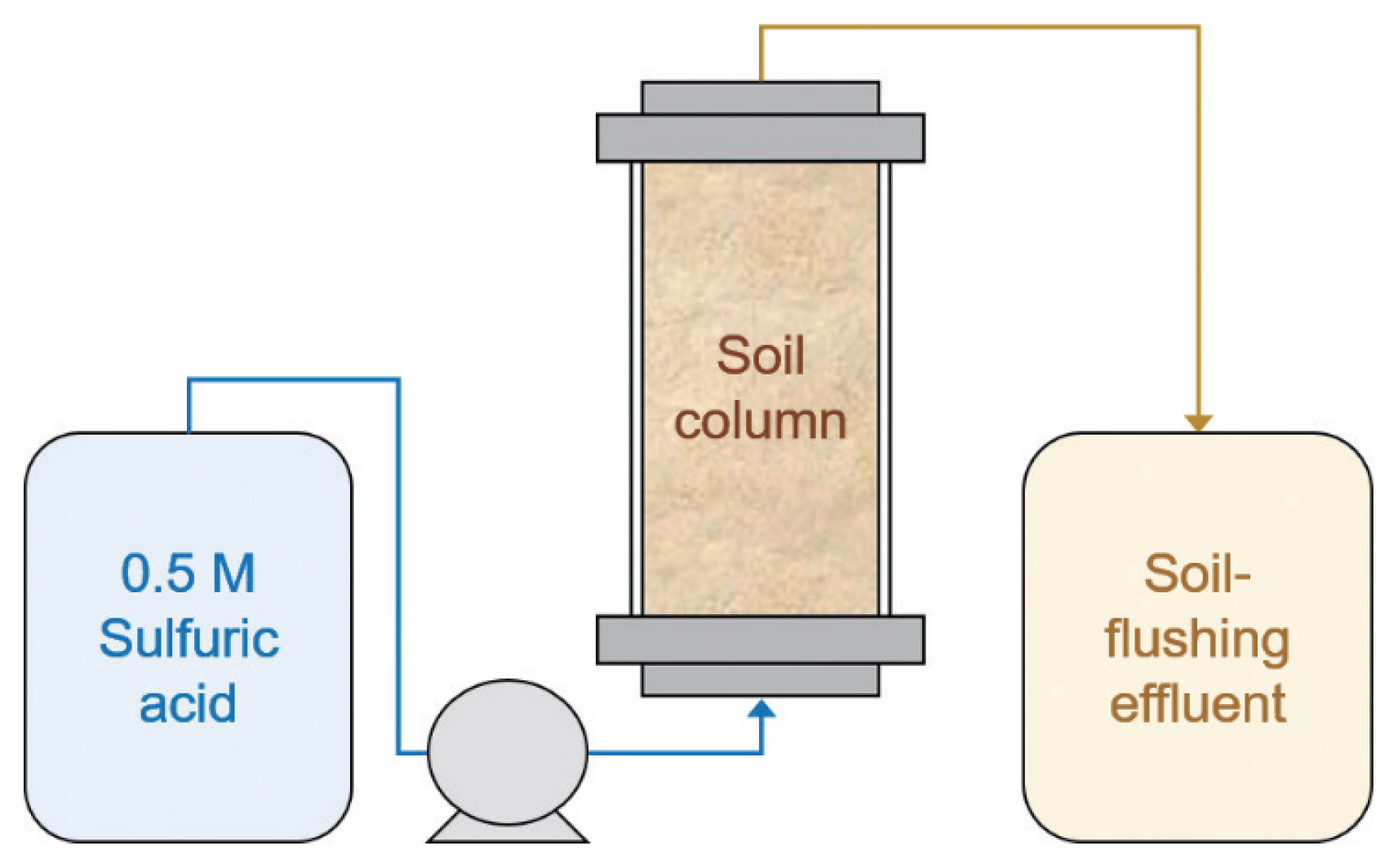
U-contaminated soil-flushing effluent was generated during a soil-flushing process using 0.5 M H2SO4 eluent in a column filled with 20 kg of U-contaminated soil (Fig. 1). A 0.5 M H2SO4 solution was prepared by mixing concentrated H2SO4 and deionized (DI) water. The DI water was obtained from a water purification system (Aqua MAX Basic 360; Younglin). After the soil column was filled with U-contaminated soil, 0.5 M H2SO4 solution was slowly flowed upward at a linear velocity of 0.23 cm/min using a peristaltic pump. An 85 L of 0.5 M H2SO4 solution was passed through the soil column for 24 hours. The solution that passed through the column was then used in purification experiments.
3. Purification of the U-Contaminated Soil-Flushing Effluent
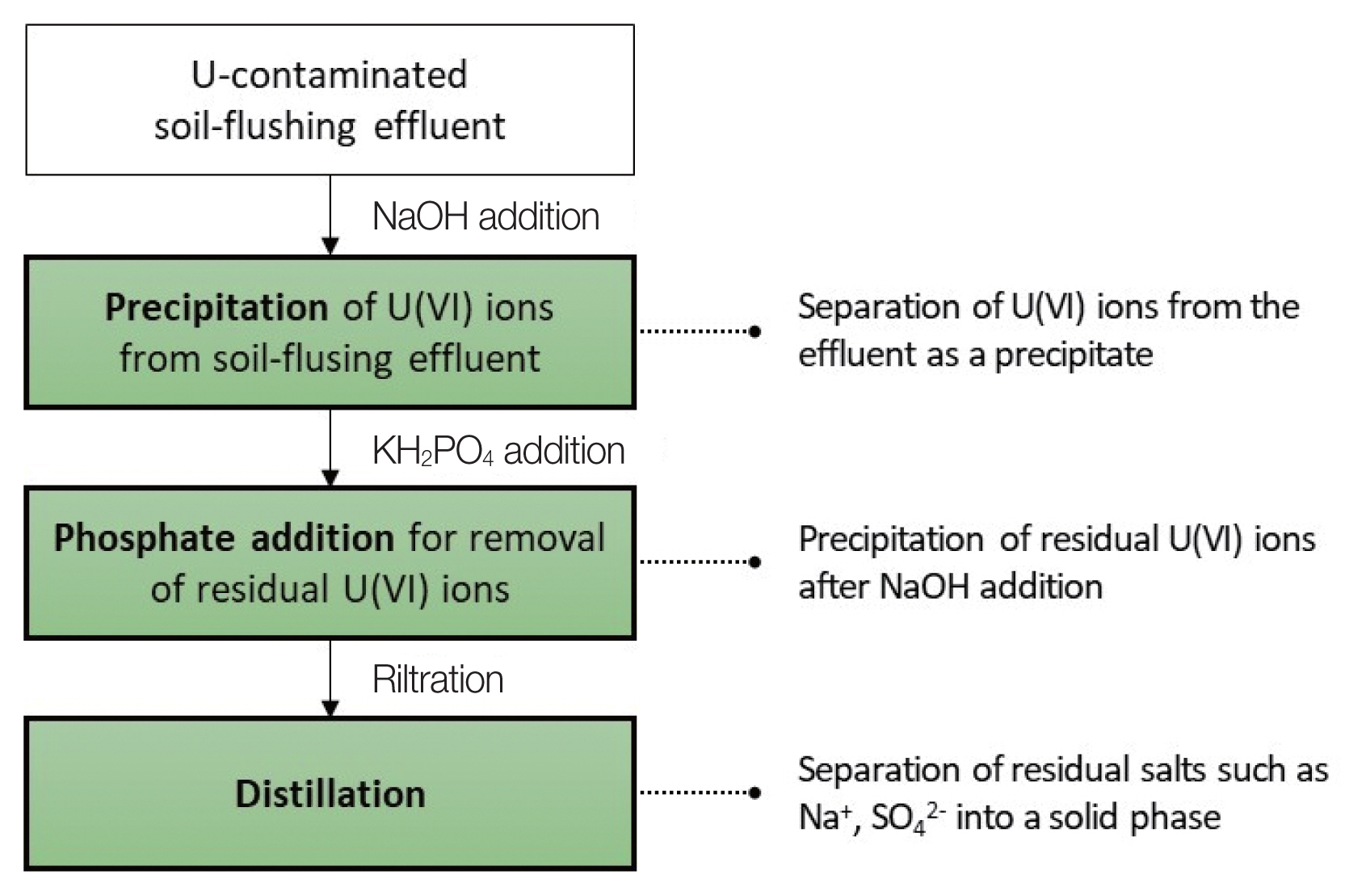
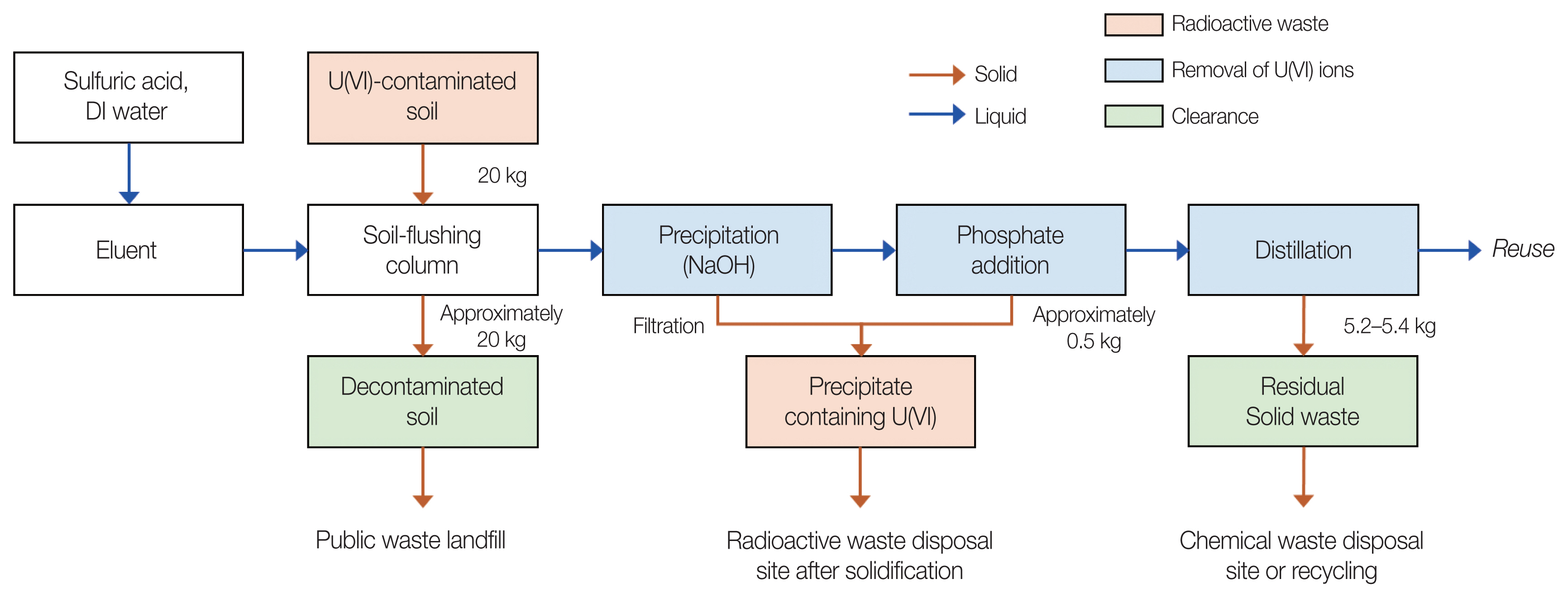
Fig. 2 shows the steps of the purification process of the U-contaminated soil-flushing effluent focused on in this study. To precipitate U(VI) ions in the soil-flushing effluent, the U-contaminated soil-flushing effluent with an initial pH of 0.56 was neutralized using NaOH powder. In order to investigate the precipitation behavior of U(VI) ions according to pH, the pH was adjusted in the range of 2 to 7. In addition, KH2PO4 powder was added to soil-flushing effluent neutralized to pH 7.4 at concentrations of 2, 5, and 10 mM to precipitate residual U(VI) ions. After precipitation and phosphate addition, 100 mL of the supernatant of the solution was aliquoted and filtered through a 0.2 μm sylinge filter (Whatman). The filtrate was poured into a two-neck glass reactor equipped with a condenser and, then, distilled at 185 °C using a heating mantle (MS-DMSB; Misung Scientific).
4. Analytical Methods
To accurately analyze the U(VI) concentration in the soil, the soil was dried at 60 °C and, then, finely pulverized using a mixer mill (MM400; Retsch). The U(VI) concentration of the pulverized soil was measured with an energy-dispersive X-ray fluorescence (ED-XRF) spectrometer (XEPOS; Spectro). The pH was measured using a pH meter (OrionStar T910; Thermo Scientific). A standard solution with a concentration range of 1 to 200 ppm was prepared using 0.5 M H2SO4 as a blank solution, and a calibration curve for U(VI) of portable XRF was produced (R2=0.9979). The ion concentration in the experimental solution was analyzed using a portable XRF spectrometer (X-200; SciAps) after the sample suspension was filtered through a 0.2 μm filter (syringe filter). A U concentration of 81 mg/L was converted to 1 Bq/g.
Results and Discussion
1. Decontamination of U-Contaminated Soil
Table 1 shows the changes in the U concentration in the soil before and after soil-flushing, the pH of the soil-flushing effluent, and the concentrations of U(VI) and iron (Fe) ions. During the soil-flushing process using 0.5 M H2SO4 as an eluent, the average U(VI) concentration of the U(VI)-contaminated soil decreased from 23.2 to 0.23 Bq/g. Therefore, the U-decontaminated soil was confirmed to have been decontaminated to a clearance level of less than 1 Bq/g [26] through the soil-flushing process. The volume of effluent generated from the soil-flushing process was 132.1 L, the effluent pH was 0.56, and its U(VI) and Fe ion concentrations were 197.6 and 590.4 mg/L, respectively.
2. Purification of the U-Contaminated Soil-Flushing Effluent
1) Neutralization–precipitation
Upon neutralization of the soil-flushing effluent using NaOH powder, U(VI) ions can be precipitated as a solid phase in the form of a hydroxide. The following equations represent precipitation reactions between
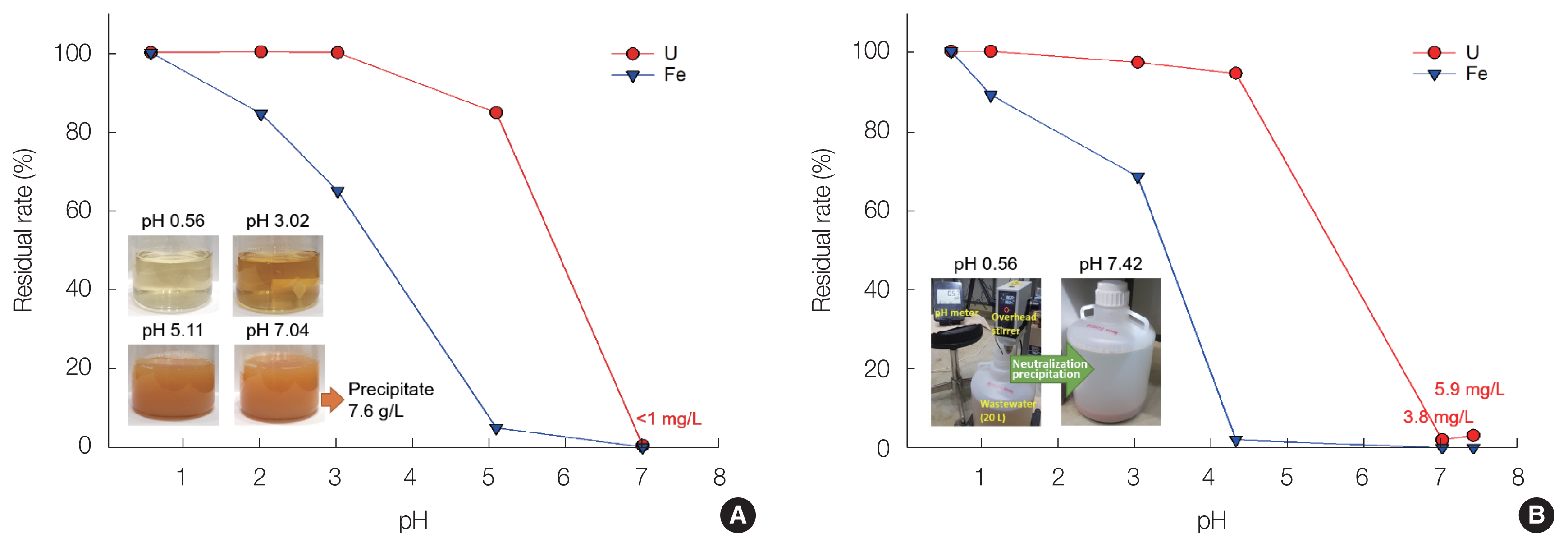
In addition to these chemical reactions, it is possible to form U(VI) precipitates such as Na4(UO2)6(SO4)3(OH)10(H2O)4 containing metal ions and sulfates [28]. Fig. 3 shows the residual rate (%) of U(VI) and Fe ions as a function of the pH during neutralization of the soil-flushing effluent. Fe ions were easily precipitated at lower pH levels compared with U(VI) ions and were steadily precipitated as the pH increased. However, U(VI) ions started to precipitate at pH 3, and the precipitation reaction was clearly observed visually at pH >5. However, in the neutralization treatment of 100 mL of soil-flushing effluent, the U(VI) ion concentration was <1 mg/L at pH 7.04 (Fig. 1A), whereas, in the case of 20 L of soil-flushing effluent, it was found to be 3.8 and 5.9 mg/L (0.05 and 0.07 Bq/g) at pH 7.01 and 7.42, respectively (Fig. 1B). The residual U(VI) concentration of 0.05 to 0.07 Bq/g in the solution is sufficiently low for it to be discharged into the surrounding environment; however, U(VI) can accumulate in the solids in the distillation process for water reuse. Therefore, in order to prevent the generation of radioactive solid waste including U(VI) in the distillation process, it is necessary to remove U(VI) ions that may remain after the precipitation process using NaOH. Meanwhile, the amount of sediment generated during the neutralization treatment of 100 mL of soil-flushing effluent was estimated to be 7.6 g/L. The sediment will contain silicon and aluminium in addition to U(VI) and Fe [29].
2) Phosphate addition
The solubility of uranyl phosphates is very low; thus, phosphate is applied to the precipitation of U(VI) ions in solution as a treatment method for secondary wastewater [30]. Therefore, phosphate addition has been considered for removal of U(VI) ions remaining after the precipitation process using NaOH. The reaction between a uranyl ion and phosphate in the soil-flushing effluent can proceed as follows [31, 32]:
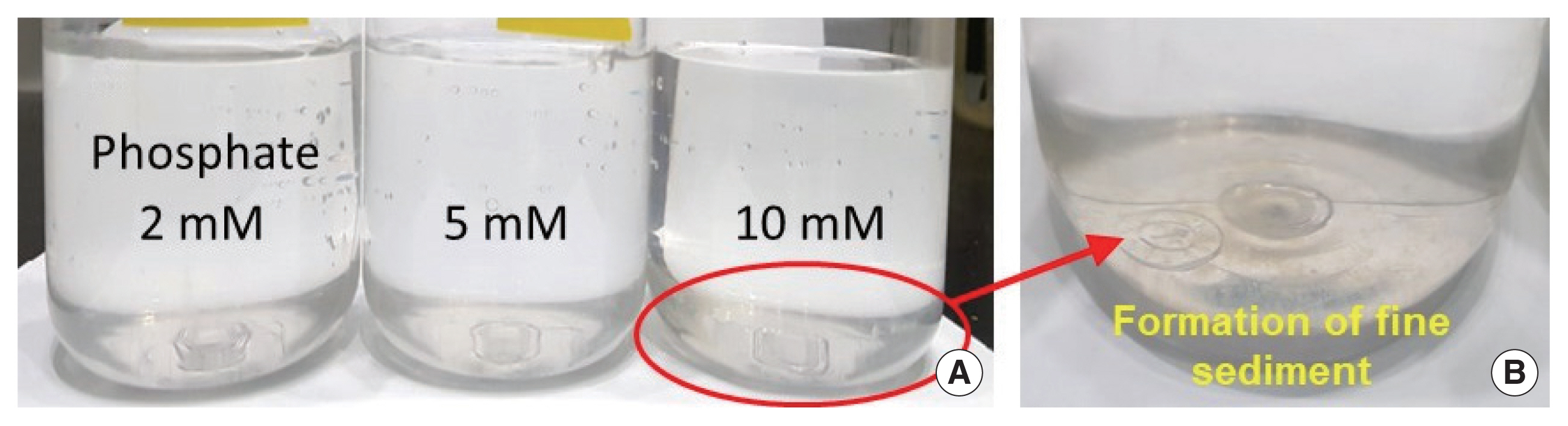
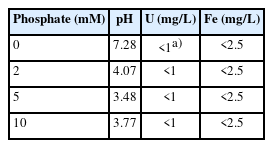
The molecular formula of uranyl phosphates can be expressed as MUO2PO4, where the cation corresponding to M can be Na+, K+, NH4+, and other cations [33]. Foster et al. [32] experimentally confirmed that the optimal pH range for the formation of uranyl phosphates is 6.0 to 6.5. Therefore, in the present study, KH2PO4 powder was added to remove U(VI) ions remaining after neutralization of the soil-flushing effluent using NaOH powder. At pH 7.42, the concentration of U(VI) ions remaining in 20 L of the soil-flushing effluent was 5.9 mg/L. However, when 2, 5, or 10 mM phosphate was added to this effluent, the U(VI) ion concentration was found to be <1 mg/L. In addition, we visually observed that a fine precipitate was formed when 2 to 10 mM phosphate was added (Fig. 4).
3) Distillation
Table 2 shows the pH and the U(VI) and Fe concentrations of the distillate of soil-flushing effluent treated by neutralization and phosphate precipitation. The pH of all the distillates was lower than that before distillation; this phenomenon is attributed to the effect of sulfate and phosphate ions transferred to the distillate and to the effect of CO2 in the air [34, 35]. The U(VI) and Fe ion concentrations were confirmed to be <1 mg/L and <2.5 mg/L, which are the respective detection limits. In particular, the U(VI) ion concentration of the effluent without phosphate addition decreased from 5.9 mg/L before the distillation process (Table 3) to <1 mg/L after the distillation process.
pH and U(VI) and Fe Concentrations of Distilled Water in Neutralization/Phosphate Precipitation Solutions with Various Concentrations

pH and U(VI) and Fe Ion Concentrations of Soil-Flushing Effluent after the Addition Of Various Concentrations of Phosphate
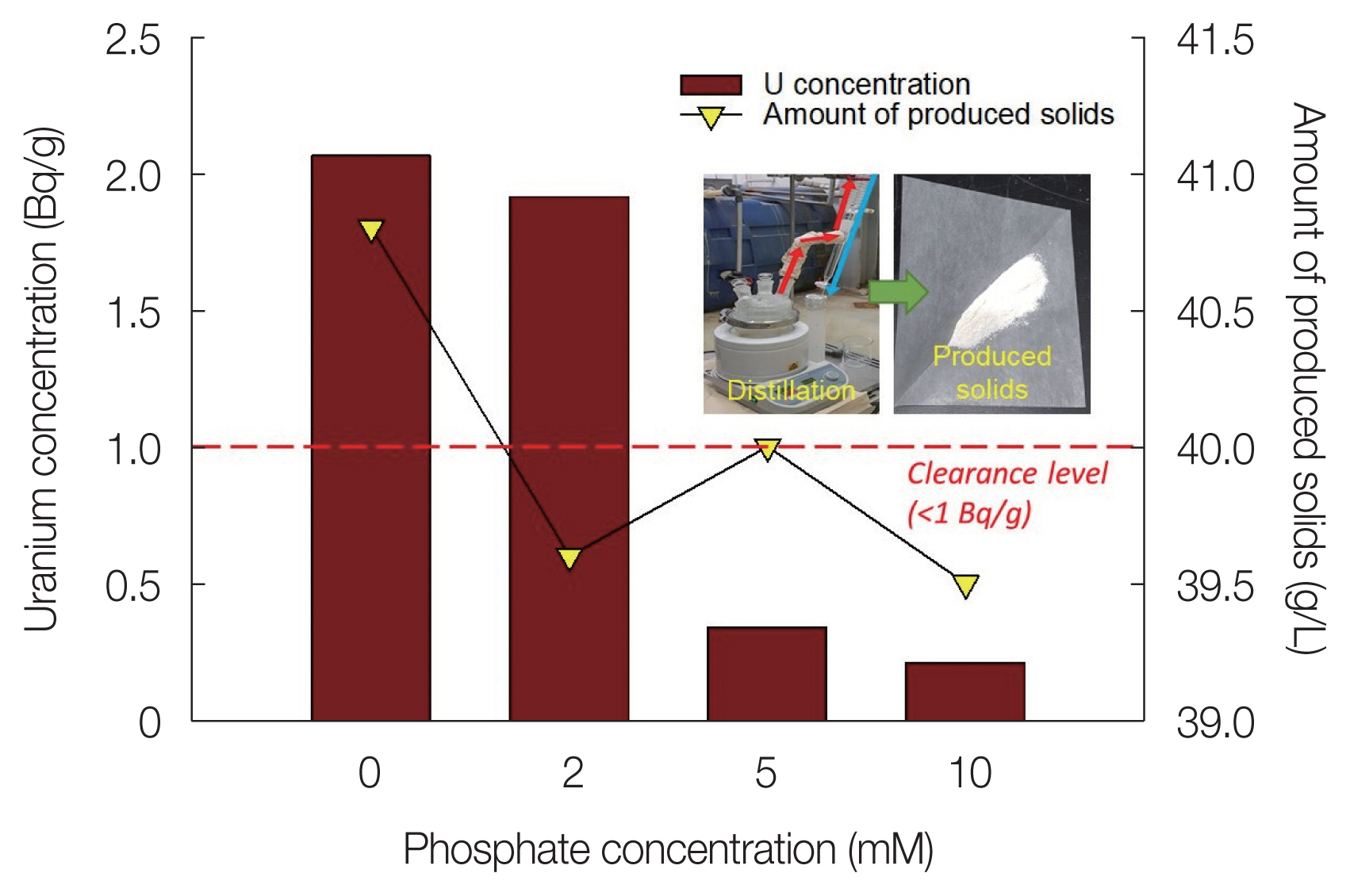
Fig. 5 shows the U concentration and the amount of produced solids of the distillation residue depending on the phosphate concentration. The U(VI) concentration of the solid produced from the distillation of the effluent without phosphate addition was found to be 2.06 Bq/g. In the case where 2 mM phosphate was added, the U(VI) ion concentration in the effluent was lowered to <1 mg/L (Table 3). However, the U concentration in the distillation residue was 1.91 Bq/g; thus, the clearance level (<1 Bq/g) was not achieved. When the phosphate concentration was 5 or 10 mM, the U concentration of the distillation residue was 0.34 or 0.21 Bq/g, respectively. This phenomenon is expected because the U(VI)-phosphate precipitation is promoted as the phosphate concentration increases [36]. Therefore, the results of this experiment suggest that phosphate should be added at a concentration of 5 mM or greater to stably precipitate U(VI) ions remaining after neutralization of the soil-flushing effluent with phosphate. However, the amount of solids produced by distillation tended to decrease as the phosphate concentration increased, which we attributed to greater phosphate concentrations resulting in more cations, including U(VI) in the effluent, being removed as precipitates before distillation.
3. Proposed Process for Purifying the U-Contaminated Soil-Flushing Effluent
A schematic of the proposed process for purifying the U(VI)-contaminated soil-flushing effluent is illustrated in Fig. 6. Assuming that 20 kg of U-contaminated soil is decontaminated in one cycle, we propose the following soil-flushing effluent purification process on the basis of the results of the present study: Because there is almost no soil leaking during the column-type soil-flushing process, if U-contaminated soil is decontaminated, the amount of soil wastes subject to clearance is ~20 kg. Most of the U(VI) ions are removed via the neutralization–precipitation process using NaOH, and the radioactive waste generated at this time is estimated to be 0.5 kg. In order to promote the precipitation reaction of U(VI) ions after the precipitation process as well as to stably precipitate U(VI) ions that may remain in the precipitation process, 5 mM phosphate is added. On the other hand, the phosphate concentration was determined based on the results of this study, and it is necessary to optimize it according to the state of the wastewater and the process performance. The solution from which U(VI) ions are precipitated is reused to prepare an eluent for further soil-flushing via a distillation process. Radioactive waste generated in the proposed process is only a sediment that includes U(VI) generated during precipitation and/or phosphate addition; the residual solid waste from the decontaminated soil and distillation can be classified as clearance.
Conclusion
In this study, U-contaminated soil-flushing effluent was purified by a precipitation–distillation process. Most of the U(VI) ions in the liquid waste generated by the soil-flushing process were removed by a neutralization–precipitation reaction using NaOH powder. The remaining U(VI) ions were effectively precipitated by phosphate addition. The effluent from which U(VI) ions were removed was distilled for reuse as a soil-flushing eluent. The U concentration of residual solid waste generated during the distillation process was found to be <0.34 Bq/g, indicating that it satisfies the clearance level. Therefore, these results suggest that more than 84% of the solid waste generated in the soil-flushing effluent purification process can be classified as clearance. These results indicate that the purification method using the precipitation–distillation process proposed in the present study effectively removes U(VI) ions from U(VI)-contaminated soil-flushing effluent and that, furthermore, most of the solid waste generated in the purification process is subject to clearance. Overall, the proposed process has potential as a soil-flushing effluent treatment method to reduce the amount of radioactive waste generated.
Acknowledgements
This work was supported by a research grant from the Korea Atomic Energy Research Institute (KAERI) (Grant No. 521220-22, South Korea).
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Ethical Statement
According to Bioethics and Safety Act in Republic of Korea, neither approval from the ethics committee nor informed consent from the study populations is required for this study.
Author Contribution
Conceptualization: Lee HK, Kim I. Methodology: Lee HK, Kim I. Formal analysis: Chang S. Supervision: Jeon H. Resources: Chang S. Funding acquisition: Jeon H. Project administration: Park S. Investigation: Park W. Writing - original draft: Lee HK. Writing - review and editing: Kim I, Yoon IH. Approval of final manuscript: all authors.