A Review of Organ Dose Calculation Methods and Tools for Patients Undergoing Diagnostic Nuclear Medicine Procedures
Article information
Abstract
Exponential growth has been observed in nuclear medicine procedures worldwide in the past decades. The considerable increase is attributed to the advance of positron emission tomography and single photon emission computed tomography, as well as the introduction of new radiopharmaceuticals. Although nuclear medicine procedures provide undisputable diagnostic and therapeutic benefits to patients, the substantial increase in radiation exposure to nuclear medicine patients raises concerns about potential adverse health effects and calls for the urgent need to monitor exposure levels. In the current article, model-based internal dosimetry methods were reviewed, focusing on Medical Internal Radiation Dose (MIRD) formalism, biokinetic data, human anatomy models (stylized, voxel, and hybrid computational human phantoms), and energy spectrum data of radionuclides. Key results from many articles on nuclear medicine dosimetry and comparisons of dosimetry quantities based on different types of human anatomy models were summarized. Key characteristics of seven model-based dose calculation tools were tabulated and discussed, including dose quantities, computational human phantoms used for dose calculations, decay data for radionuclides, biokinetic data, and user interface. Lastly, future research needs in nuclear medicine dosimetry were discussed. Model-based internal dosimetry methods were reviewed focusing on MIRD formalism, biokinetic data, human anatomy models, and energy spectrum data of radionuclides. Future research should focus on updating biokinetic data, revising energy transfer quantities for alimentary and gastrointestinal tracts, accounting for body size in nuclear medicine dosimetry, and recalculating dose coefficients based on the latest biokinetic and energy transfer data.
Introduction
Exponential growth has been observed in nuclear medicine procedures worldwide in the past decades. The considerable increase is attributed to the advances in positron emission tomography and single photon emission computed tomography [1], as well as the introduction of new radiopharmaceuticals [2–4]. The annual per capita effective dose from nuclear medicine procedures in the United States was about 0.14 mSv in 1980, which doubled to 0.32 mSv in 2016 [5, 6]. Although nuclear medicine procedures provide undisputable diagnostic and therapeutic benefits to patients, the substantial increase in radiation exposure to nuclear medicine patients raises concerns about potential adverse health effects and calls for the urgent need to monitor exposure levels involved in the procedures.
Direct measurement of internal organ doses from radionuclides distributed within a patient’s anatomy is not feasible. Hence, nuclear medicine dosimetry is based on a computational method, called the Medical Internal Radiation Dose (MIRD) formalism [7]. According to the method, organ-level dosimetry for nuclear medicine patients requires two types of quantification: biokinetic distribution of administered radionuclides in organs and tissues, called source regions, and energy transfer from source regions to organs and tissues of interest, called target regions. There are two different approaches to estimating them. In the first approach, called model-based dosimetry, these quantities can be derived using biokinetic models and computerized anatomy models representing standard humans. Biokinetic models are composed of a series of mathematical equations that describe the time-dependent transfer of radionuclides injected into human body through different paths. Computerized anatomy models, called computational human phantoms, describe the internal organ and tissue structures of the human anatomy by using different modeling tools such as mathematical equations, volume pixels, or surfaces. Energy transfer from source to target regions is calculated using computational human phantoms combined with Monte Carlo radiation transport methods. The model-based dosimetry methods allow for creating dose calculation tools based on a library of biokinetic data and pre-calculated dose tables. Second, a more advanced and individualized approach to organ-level dose estimation, called patient-specific dosimetry, is based on the functional and anatomical imaging of a patient. These image-based data can serve as biokinetic and standard anatomy models in model-based dosimetry. Such methods can provide much more accurate dosimetry compared to model-based dosimetry, which may fail to consider individual variability in biokinetic and anatomical data. Patient-specific dosimetry is performed more for therapeutic nuclear medicine procedures than diagnostic ones and requires expensive imaging data and extensive computation time.
The current review focuses on the model-based dosimetry approach. The review will cover the following topics: MIRD formalism, biokinetic data, human anatomy models focusing on internal dosimetry, energy spectrum data of radionuclides, and existing organ dose calculation tools for nuclear medicine procedures. The review will be finished with comments on major research needs in nuclear medicine dosimetry.
Nuclear Medicine Dosimetry Methods
Dosimetry methods for nuclear medicine patients are based on the MIRD formalism, which requires the following components: biokinetic data, energy transfer data-based on computational human anatomy model combined with Monte Carlo radiation transport techniques, and radionuclide energy spectrum. The MIRD formalism and its components will be discussed in detail in the following sections.
1. MIRD Formalism
The fundamental calculation methods for internal dosimetry were established in the MIRD formalism introduced in the 1960s [8] and have been updated to date [7, 9–11]. Although the units of basic quantities have been slightly changed, the definition of the major quantities largely stays the same. It has been noted that the naming of the quantities differs between MIRD and International Commission on Radiological Protection (ICRP). The current review uses the notation and naming provided in the MIRD pamphlet No. 21 [11].
When the source and target regions are defined in human anatomy, absorbed fraction (AF), the fraction of the particle energy emitted from a source region rS to that is deposited in a target region rT, can be calculated using computational human phantoms combined with Monte Carlo radiation transport methods, as denoted in the following equation:
AF must be calculated for different particles (e.g., photon, electron) when a given radionuclide contains multiple types of particles in its energy spectrum. Specific absorbed fraction (SAF; kg−1) is then calculated through dividing AF values by the mass of a target region mrT as shown in the following equation:
Then, S (mGy/MBq-s), absorbed dose to a target region per unit cumulated activity in a source region, can be derived from the photon and electron SAFs (kg−1) using the following equation:
where ni is the probability of emission of the radiation type i with the energy Ei of a given radionuclide, and Φ(rT ← rS) is the SAF for a source region rS and a target region rT. Once a library of S values is calculated for different source and target regions and radionuclides, organ absorbed dose to a target region rT can be calculated using the following equation:
where Ã(rS) is the time-integrated or cumulated activity of the radiopharmaceutical in the source region rS, and S(rT ← rS) is the S value to the target region rT per unit activity present in the source region rS.. When multiple source regions are involved, absorbed doses from different source regions are summed to estimate a total absorbed dose to a target region.
To make dose calculation process more efficient for nuclear medicine procedures, absorbed doses per unit administered activity (mGy/MBq), D(rT)/A0, also called absorbed dose coefficients, to target region rT can be calculated for different radiopharmaceuticals using the following equation:
where Ã(rS)/A0 is the time-integrated activity coefficient (TIAC), previously named residence time, for source region rS and S(rT ← rS) is the S value for the source region rS and target region rT for a given radionuclide.
2. Biokinetic Data
Biokinetic data can be developed for different purposes, such as radiation protection for the public and dosimetry for nuclear medicine patients. Biokinetic models and data created for radiation protection are thoroughly reviewed elsewhere by Paquet [12]. The current review will focus on the biokinetic data developed for nuclear medicine procedures. Major contributions to biokinetic modeling have been made by the MIRD Committee of the United States Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the Oak Ridge National Laboratory (ORNL). The ICRP has also supplied biokinetic and dosimetric data for radiopharmaceuticals frequently used in nuclear medicine procedures. The current review focuses on a series of ICRP Publications on biokinetic data for nuclear medicine dosimetry.
ICRP Publication 53 [13] was published in 1988 and provides the biokinetic data and absorbed dose coefficients (absorbed dose per unit administered activity) for 120 radiopharmaceuticals composed of 71 radionuclides of 34 elements. A library of AFs calculated from the MIRD stylized hermaphrodite phantom [14] was used to calculate S values, which were then applied to deriving absorbed dose coefficients. The organ masses of 1, 5, 10, and 15 years and adult reference individuals reported in ICRP Publication 23 were adopted for calculating SAFs and S values [15].
Since ICRP Publication 53, a series of addenda have been published when biokinetic data for new radiopharmaceuticals were available. The first addendum to Publication 53 was included in ICRP Publication 62 [16]. Biokinetic and dosimetric data for six new radiopharmaceuticals were provided in the report: 3H neutral fat and free fatty acids, 14C neutral fat and free fatty acids, 68Ga ethylenediaminetetraacetic acid, 99mTc hexamethylpropyleneamine oxime (HM-PAO; Ceretec, GE Healthcare), 99mTc mercaptoacetyltriglycine, and 99mTc methoxyisobutyl isonitrile. The second addendum was published in ICRP Publication 80 [17]. The biokinetics and dosimetry data for 10 new radiopharmaceuticals and the recalculations of dosimetric data for the 19 most frequently used radiopharmaceuticals previously included in Publication 53 are provided in the addendum. The 10 new radiopharmaceuticals include (Methyl-11C) thymidine; (2-11C) thymidine; 14C urea (including carbon dioxide and bicarbonate); 15O water; 99mTc human immunoglobulin (HIG), pertechnegas, technegas, and tetrofosmin; and 111In HIG and octreotide. The third addendum to Publication 53 was published as ICRP Publication 106 [18]. The publication provides biokinetic and dosimetric data for 33 new radiopharmaceuticals. It also includes recommendations for breastfeeding mothers undergoing nuclear medicine procedures. Addendum 4 was released in 2013 only through the ICRP website [19]. The report provides the biokinetic models, absorbed dose, and effective dose coefficients for five new radiopharmaceuticals (18F-fluoro-ethyl-tyrosine, 18F-fluorothymidine, 18F-choline, 11C-raclopride, and 18F-fluoride) and two corrections to Publication 106.
More recently, ICRP published a compendium in Publication 128 [20]. The publication contains biokinetic and dosimetric data compiled from the previous Publications 53, 80, and 106, and amendments and corrections. The report also provides new data for 82Rb-chloride, iodide (123I, 124I, 125I, and 131I), and 123I- labeled 2β-carbomethoxy 3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane (FP-CIT).
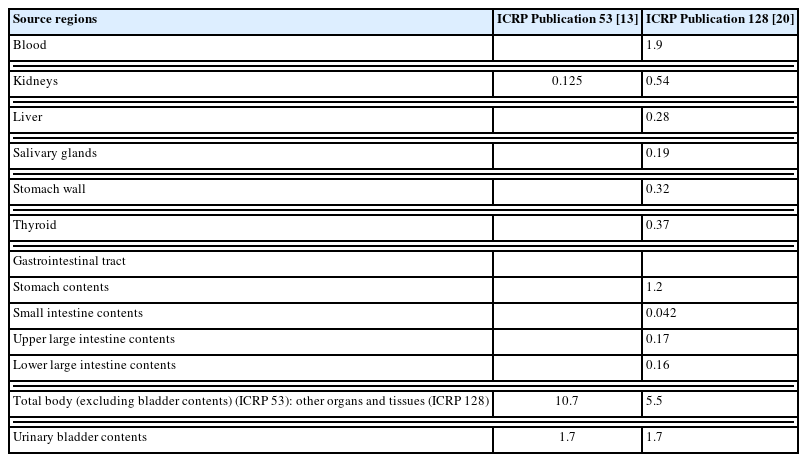
Biokinetic data provided in ICRP Publications are composed of the fraction of the injected activity in a source organ FS, biological half-time T (hr), the fraction of FS eliminated with half-time T, and TIAC (hr). Absorbed dose coefficients (absorbed dose per unit administered activity) (mGy/MBq) for a series of target regions and effective dose per unit administered activity (mSv/MBq) are provided by phantom age: 1, 5, 10, 15 years, and adult. For example, Table 1 compares the two sets of biokinetic data for 124I intravenous administration between ICRP Publication 53 [13] and 128 [20]. Note that ICRP 128 provides more TIACs for more detailed organs and tissues compared to ICRP 53.
3. Human Anatomy Models for Internal Dosimetry
One of the key components in dosimetry for nuclear medicine patients is the library of S values derived from SAFs and the energy spectrum of radioisotopes as described in Equation (3). As mentioned above, SAFs are derived from AFs which are calculated from computational human phantoms combined with Monte Carlo radiation transport methods. Computational human phantoms have evolved from simplified stylized (or mathematical or MIRD-type) phantoms through more realistic voxel (or tomographic) phantoms to flexible hybrid (or boundary representation [BREP] or mesh) phantoms. The evolution has been driven to achieve anatomical realism and geometrical flexibility eventually for increased dosimetric accuracy. Whenever a new generation of computational human phantoms was introduced, more detailed and accurate S value libraries were calculated. Since the general characteristics of computational human phantoms are already reviewed thoroughly by other authors [21–24], the current review focuses on applying computational phantoms to nuclear medicine dosimetry and dosimetry improvement achieved by their evolution.
1) Stylized phantoms
Stylized phantoms, the first-generation computational human phantoms, were developed solely for internal radiation dosimetry at the ORNL [14]. Anatomical structures in the human body were described using mathematical equations. Arms were included in the torso region since they did not have any dosimetric impact when the radiation source is internally distributed. The adult hermaphrodite phantom [14, 25] was used to calculate a library of AFs for photons by using Monte Carlo radiation transport techniques. Following the adult phantom, a series of pediatric stylized phantoms [26] were developed and adopted to calculate a library of pediatric AFs and SAFs [27]. To improve the anatomical accuracy of the simplified head model in the stylized phantom series, Bouchet et al. [28] developed a detailed stylized head model including eight subregions: the caudate nuclei, cerebellum, cerebral cortex, lateral ventricles, lentiform nuclei, thalami, third ventricle, and white matter. They calculated S values for 24 radionuclides used in clinical or investigational brain studies. A series of stylized pregnant woman phantoms were later developed by Stabin et al. [29] and were used to calculate a library of SAFs of photons.
Other than the phantoms based on the Caucasian reference data, Park et al. [30] developed a Korean adult stylized phantom by adjusting the ORNL adult male stylized phantom. To improve the accuracy of internal dosimetry for weakly penetrating electrons, they divided the wall of the esophagus, stomach, colon, and urinary bladder into the mucosal layer and residual wall. They found the AFs estimated from the MIRD schema overestimated those calculated from their improved mucosa wall of the urinary bladder by over 30%.
2) Voxel phantoms
Stylized phantoms provided mathematical flexibility that allowed for modifying organ shape and size. However, one of the critical dosimetric limitations in the phantom format was the simplified anatomical structures compared to those of real human bodies. To improve anatomical realism, a new generation of computational human phantoms, called voxel (or tomographic) phantoms, was introduced and adopted for internal dosimetry calculations. Since the introduction of the first voxel phantoms, baby and child, created by Zankl et al. [31], there has been a surge of voxel phantoms developed by different researchers and used for internal dosimetry calculations. The results have been compared with those from the stylized phantoms to evaluate the potential dosimetric improvement achieved by voxel phantoms.
Following baby and child, Zankl and Wittmann [32] created an adult voxel phantom, Golem, using a whole-body computed tomography image of a 38-year-old male. Using the baby, child, and Golem voxel phantoms, Petoussi-Henss and Zankl [33] calculated SAFs for various source and target organs, and compared the results with those from the 5- and 10-year-old MIRD stylized phantoms. They concluded that for some source-target organ combinations, discrepancies in SAFs were up to one or two orders of magnitude depending on photon energy and the location and masses of the organs involved. Smith et al. [34] later calculated the SAFs for additional organs using the Golem voxel phantom and compared the results with those from the traditional MIRD-type adult phantom. They suggested that individual tissue doses from voxel phantoms could differ substantially from those from the MIRD-type phantoms, but the effective dose did not change greatly. Zankl et al. [35] later used seven male and female adult voxel phantoms and calculated SAFs. The authors observed large variations in photon SAFs among the voxel phantoms up to orders of magnitude for very low photon energies. They also found even larger differences in SAFs between the voxel phantoms and the MIRD-type phantoms since the interorgan distances tend to be larger in the MIRD-type phantoms than in reality. Petoussi-Henss et al. [36] also published a paper on patient dose from radiopharmaceuticals using four male and three female voxel phantoms. They concluded that dose differences among the voxel phantoms could amount to a factor of three.
The Zubal phantom [37, 38] is one of the early voxel phantoms. It was developed by manually segmenting 45 internal human organs from magnetic resonance images to simulate gamma camera imaging for nuclear medicine patients. Chiavassa et al. [39] calculated S values using the Zubal voxel phantom combined with three Monte Carlo radiation transport codes, EGS4, MCNP4c2, and MCNPX2.5e. They found that the difference in S values between EGS4 and MCNPX2.5e was less than 10% for all target and source regions they included in the study. Yoriyaz et al. [40, 41] calculated AFs, SAFs, and S values using the Zubal phantom and compared the results with those from the ORNL adult phantom. They found considerable discrepancies in some source-target organ pairs due to differences in the organ mass between the two phantoms and the occurrence of close contact among organs in the Zubal phantom, which cannot be realistically described in the stylized phantom. They proposed using voxel phantoms for patient-specific dose estimates in internal emitter therapy.
Xu et al. [42] developed a whole-body voxel phantom, called VIP-Man, using transversal color photographic images obtained from the National Library of Medicine’s Visible Human Project. Chao and Xu [43] used the VIP-Man voxel phantom to calculate SAFs for electrons uniformly distributed in 26 organs. The phantom provided high voxel resolution, 0.33 mm× 0.33 mm×1 mm, which enabled the modeling of skin, eye lenses, and mucosa layer in the gastrointestinal tract (esophagus, lower large intestine, upper large intestine, stomach, and small intestine), and red bone marrow distributed within the spongiosa bone regions. These small structures were not properly represented in the stylized phantoms. The authors report that the dose to the mucosa layer is greater than that to the total wall by an order of two. Chao and Xu [44] used the high-resolution head model of the VIP-Man voxel phantom to calculate S values for 11C, 15O, 18F, 99mTc, and 123I used in brain imaging procedures. They found that the stylized head/brain phantom [28] underestimated the S values from the high-resolution voxel head phantom by up to 88%. Shi and Xu [45] from the same research team later developed a pregnant woman voxel phantom using the computed tomography images of a patient who was 30 weeks into pregnancy and used the phantom to calculate SAFs for internal photons [46]. They compared their results with those from the stylized pregnant woman phantom. Even though the two results showed general agreement for high energies, substantial differences were found for lower energy photons. The authors suggested that the main reasons for the differences were the variation of organ mass, geometry, and interorgan distance between the voxel and stylized pregnant phantoms.
Lee et al. [47] developed two Korean voxel phantoms representing an average Korean adult male. They employed one adult male voxel phantom and calculated SAFs for 37 source and 32 target organs and tissues [48]. In comparing SAFs between the Korean voxel phantom and the ORNL stylized phantom, they found that the difference in organ mass between Korean and Caucasian adult males (represented by the ORNL phantoms) was one of the key factors impacting the difference in self-SAFs. They also observed significant differences in crossfire SAFs between the two phantoms, mainly due to anatomical differences.
In the past, the ICRP had not specified a particular phantom when it published the equivalent doses for the reference male and female and effective doses for the reference person, although those data were based on various stylized phantoms. In ICRP Publication 103 [49], the commission officially adopted reference voxel phantoms as reference computational human phantoms representing the adult reference male and female for calculating equivalent doses for organs and tissues. In ICRP Publication 110 [50], the commission officially published its first adult reference male and female voxel phantoms. The phantoms were developed by modifying the Golem (male) and Laura (female) voxel phantoms [51] created at the Helmholtz Zentrum Munchen-German Research Center for Environmental Health.
As soon as the phantoms were released, Hadid et al. [52, 53] used the ICRP adult voxel phantoms to evaluate the impact of the new voxel phantoms on internal dosimetry. They calculated the absorbed dose from 11 radiopharmaceuticals currently used in diagnostic nuclear medicine. They compared the results with those from the previous ICRP Publications based on the ORNL stylized phantoms. They found that the stylized phantom-based absorbed dose overestimated the values from the voxel phantoms by over 200% (urinary bladder wall). Lamart et al. [54] used the ICRP adult male and female voxel phantoms to establish a library of S values for 131I for 55 source regions and 42 target regions to study hyperthyroidism patients.
After the publication of external dose conversion coefficients based on the adult voxel phantoms [55], a comprehensive library of SAFs was calculated and published in ICRP Publication 133 [56]. The SAFs were calculated for photons, electrons, alpha particles, and fission-spectrum neutrons associated with radionuclides that decay by spontaneous fission. A total of 43 target organs and tissues were considered as target regions, and 79 source regions were involved in the SAF calculations. Due to the intrinsic limitation of voxel phantoms not accurately modeling thin radiosensitive layers, the SAF values for electron and alpha particles for the respiratory tracts were extracted from ICRP Publication 66 [57]. The SAF values were calculated using simplified mathematical human alimentary models similar to those used in ICRP Publication 100 [58] for the mucosa layers of the gastrointestinal tract, including the esophagus, stomach, small intestine, left and right large intestine, and rectosigmoid.
Following the introduction of the ICRP adult reference voxel phantoms, a series of ICRP reference pediatric phantoms were published in ICRP Publication 143 [59]: newborn, 1-, 5-, 10-, and 15-year-old male and female voxel phantoms. The phantom series was developed by modifying the pediatric hybrid phantoms developed by the University of Florida and the United States National Cancer Institute (UF/NCI) [60]. Villoing et al. [61] calculated a full library of photon and electron SAFs and S values using the ICRP pediatric voxel phantoms. Their S value library was established for 55 target and 68 source regions for 299 radionuclides frequently used in diagnostic nuclear medicine procedures. The database includes more detailed target regions than ICRP Publication 133. However, the authors still adopted the SAFs from ICRP Publication 133 for gastrointestinal mucosa layers.
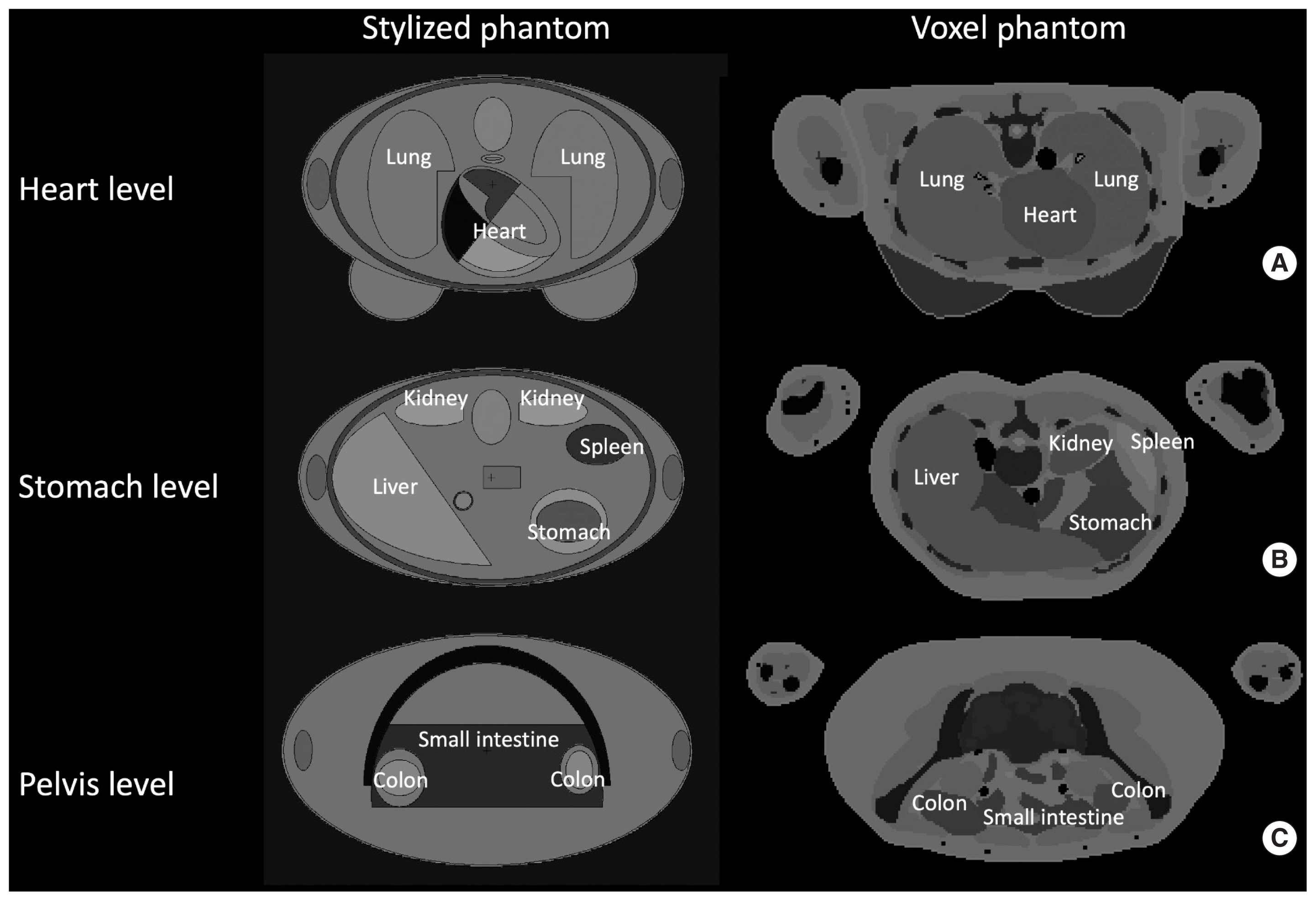
Fig. 1 compares the anatomical structures at the level of heart, stomach, and pelvis between the hermaphrodite ORNL stylized phantom and the ICRP adult female voxel phantom. The difference in interorgan distances between the two phantom types is clearly demonstrated: the organs in the voxel phantom are more in contact with each other than those in the stylized phantom.
3) Hybrid phantoms
Although the voxel phantoms have substantially improved anatomical accuracy over the stylized phantoms, they still pose some limitations. First, the voxel phantoms are fixed to the original tomographic images of patients and difficult to be modified, although limited uniform scaling is possible by adjusting voxel resolutions. Voxel tagging must be manually modified to change the shape and location of anatomical structures. Second, it is impossible to describe radiosensitive thin layers for respiratory and alimentary tracts using voxels due to their low resolution compared to the thickness of the mucosa layers. This is why ICRP Publication 133 still adopts the SAFs for respiratory and alimentary tracts from the stylized models in ICRP Publications 66 and 100, respectively. To overcome these limitations, third-generation phantoms, called hybrid (or BREP or mesh) phantoms, were developed using polygon mesh and/or non-uniform rational B-spline (NURBS) surfaces. In terms of internal dosimetry, hybrid phantoms provide two major advantages. First, their new format allows for easier and more realistic modification of the shape and location of anatomical structures compared to the voxel phantoms. By virtue of this modeling flexibility, it is possible to describe the close contact among the organs and tissues and to adjust interorgan distances as needed. Second, the most prominent advantage of the hybrid phantoms is the capability to describe very thin radiosensitive layers, such as 0.005 cm thick radiosensitive skin layer in adults [55, 62]. Several authors developed hybrid phantoms to improve dosimetric accuracy in internal dosimetry.
Lee et al. [63] created the first whole-body hybrid phantom representing a newborn baby composed of polygon mesh and NURBS surfaces. The authors later developed the adult male and female hybrid phantoms [64], and pediatric phantom series with in-between ages [60]. The full phantom series was completed by researchers at the UF/NCI, so the phantom series is called the UF/NCI phantoms. The modeling flexibility of the hybrid phantom format made it possible to adjust the volume and location of the organs and tissues of the previous pediatric voxel phantoms [65] to match those of the ICRP reference data [66]. Wayson et al. [67] calculated a library of SAFs using the UF/NCI newborn hybrid phantom, which was later extended to the phantoms with other ages for photons [68] and electrons [69]. Wayson and Bolch [70] also investigated the feasibility of personalizing internal radiation dose estimates for individual patients by modifying the dose estimates calculated from reference phantoms based on morphometric data of patients. They observed average gains in dosimetric accuracy of about 6% when sitting height and waist circumference were used for scaling reference phantoms. Lamart et al. [71] calculated S values for 131I distributed within the thyroid of the UF/NCI adult hybrid phantoms and compared the results with those from the ICRP adult voxel phantoms [56]. They found that the ratio of the S values from the UF/NCI phantoms to those from the ICRP phantoms varied from 0.4 to 2 (adult male) and from 0.3 to 1.4 (adult female), mainly due to differences in organ placement and shapes between the two phantom series. Villoing et al. [72] used the UF/NCI pediatric and adult phantom series to establish a comprehensive library of S values for 68 source regions and 55 target regions for 299 radionuclides. Villoing et al. [73] also developed detailed Korean head hybrid phantoms and calculated photon and electron SAFs, and then calculated S values for major radiopharmaceuticals used in diagnostic nuclear medicine procedures for the brain [74].
Shi et al. [75] developed a series of hybrid phantoms, called BREP phantoms, representing reference pregnant females at 3-, 6-, and 9-month gestation periods to calculate SAFs for internal photon emitters. Thanks to the flexible polygon mesh format, the authors adjusted the volume of organs to match the ICRP reference data [66]. They compared the resulting SAFs with those calculated from the ORNL stylized phantoms. They found one or two orders of differences occurring for low-energy photons, and the differences for more energetic photons are much smaller. They report that these differences are believed to be caused by the different anatomical shapes of the organs involved.
More recently, ICRP adult mesh phantoms, called mesh-type reference computational phantoms (MRCPs) [76], were developed by converting the ICRP adult male and female voxel phantoms [50] into polygon meshes as well as tetrahedral meshes. The MRCPs include all source and target regions required for estimating effective doses defined in ICRP Publication 103. The phantoms also feature the micrometer-thick radiosensitive target layers in the respiratory and alimentary tracts, skin, and urinary bladder. These are much more realistic than the previous stylistic models adopted in ICRP Publications 66 [57] and 100 [58]. In comparing SAFs between the MRCPs and the ICRP adult voxel phantoms, significant discrepancies were observed in the following cases. First, the MRCP-based SAFs were smaller when the red bone marrow is a target region because the spongiosa structure in the ICRP voxel phantoms is not fully covered by the outside cortical bone layers, especially in the ribs where a substantial portion of red bone marrow is distributed. The problem was caused by the modeling limitation of voxel phantoms, where the cortical bone layers were hand-drawn slice by slice from the top of the head to the bottom of the feet. Second, some crossfire SAFs from the MRCPs were smaller than those from the voxel phantoms because the close but not contact interorgan distance was not realistically modeled in the voxel phantoms.
4. Energy Spectrum of Radionuclides
The last component in nuclear medicine patient dosimetry is the probability of emission of radiation type i with the energy Ei of a given radionuclide, as shown in Equation (3). This information is used to derive S values from the SAF libraries for different radiation types. One of the most comprehensive nuclear decay datasets for radionuclides is the one published in ICRP Publication 107 [77]. The report supersedes the data published in ICRP Publication 38 [78]. It provides information on the half-lives, decay chains, and yields and energies of radiations emitted in nuclear transformations of 1,252 radionuclides of 97 elements.
Organ Dose Calculation Tools
S values derived from SAFs and radionuclide energy spectra can be used to convert cumulated activity in a source region to absorbed dose delivered to a target region according to Equation (4). However, radionuclides can be distributed across the human body in multiple source regions described by biokinetic models. Multiple target regions can be of interest in nuclear medicine procedures. When multiple source and target regions are involved, the dose calculation process may become time- and labor-intensive. To conduct this process more efficiently, a library of S values and biokinetic models can be implemented into graphical user interface-based computer programs. Users can input patient- and procedure-specific parameters and conveniently obtain organ doses delivered to target regions. Several computer programs developed for this purpose are available and widely utilized in dosimetry for nuclear medicine procedures. The current review focuses on model-based dose calculators since patient-specific dose calculation tools often used for therapeutic nuclear medicine require fundamentally different approaches based on anatomical and functional imaging. Table 2 summarizes the characteristics of the organ dose calculators in order by release year of the first versions [61, 72, 79–87].
1. SEECAL
SEECAL [79] is a Microsoft Disk Operating System (MSDOS)- based computer program. It calculates specific effective energies (SEE), later renamed S values, to specified target regions for reference male and female individuals aged newborn, 1, 5, 10, and 15 years, and adult. The program replaces a previous computer program, SEE, internally used in calculating SEE for occupational exposures by the Dosimetry Research Group at ORNL. The latest version of SEECAL is incorporated into Dose and Risk Calculation (DCAL), a radiation dose and risk calculator developed at the United States Environmental Protection Agency (EPA). SEECAL has been upgraded [79] by implementing the SAFs calculated from the reference voxel male and reference voxel female, the earlier version of the ICRP reference voxel phantoms [50]. DCAL can be downloaded from the EPA website (https://www.epa.gov/radiation/dcal-software-and-resources). Fig. 2A shows the MSDOS-based user interface of SEECAL embedded into the DCAL software.

Example user interfaces of (A) SEECAL, (B) Organ Level INternal Dose Assessment (OLINDA), (C) Internal Dose Assessment by Computer (IDAC)-Dose 2.1, (D) Society of Nuclear Medicine and Molecular Imaging (SNMMI) nuclear medicine radiation dose tool, (E) Medical Internal Radiation Dose (MIRD)-calc 1.0, (F) OpenDose, and (G) National Cancer Institute dosimetry system for Nuclear Medicine (NCINM).
2. OLINDA/EXM 2.0
Organ Level INternal Dose Assessment/EXponential Modeling (OLINDA/EXM) version 2.0 [80] is the latest version of the previous MIRDOSE [81] and later OLINDA/EXM 1.0 [82]. MIRDOSE was based on the SAFs calculated from the ORNL pediatric, adult, and pregnant woman stylized phantom series described in the previous sections. OLINDA/EXM was developed by the RAdiation Dose Assessment Resource (RADAR) Task Group of the Society of Nuclear Medicine to standardize organ-level dose calculations for diagnostic and therapeutic radiopharmaceuticals. The early version of OLINDA/EXM was based on the SAFs from the ORNL stylized phantoms. The SAFs calculated from the NURBS-type phantoms [88] based on the reference organ masses defined in ICRP Publication 89 [66] have been implemented into the latest version. Decay data for over 800 radionuclides [89] are included in OLINDA/EXM, including selected alpha emitters. Users must enter TIAC for the various source regions to calculate organ-level absorbed doses. OLINDA/EXM 2.0 is commercially available through Hermes (Hermes Medical Solution). Fig. 2B shows the graphical user interface of OLINDA 1.0.
3. IDAC
Internal Dose Assessment by Computer (IDAC)-Dose software version 1.0 was developed using the SAF library calculated from the ORNL pediatric and adult stylized phantom series. The software was updated to IDAC-Dose 2.0 [83] using the SAF library calculated by Hadid et al. [53] using the National Research Centre for Environment and Health (GSF) adult male and female voxel phantoms. The SAF dataset published in ICRP Publication 133 [56] was later implemented into IDAC-Dose version 2.1 [84]. The program uses the radionuclide decay data of ICRP Publication 107 and considers 83 source regions and 47 target regions defined in ICRP Publication 110 [50]. The effective dose calculated from the program is based on the tissue weighting factors published in ICRP Publication 103 [49]. IDAC-Dose 2.1 is available from the IDAC-Dose website (https://www.idac-dose.org/). Since the program has been developed using MATLAB graphical user interface tool, a user needs to install MATLAB Runtime before installing IDAC-Dose 2.1. Fig. 2C shows the user interfaces for input and results of IDAC-Dose 2.1.
4. SNMMI Dose Calculator
SNMMI hosts a dose calculator on their website (http://www.snmmi.org/clinicalpractice/dosetool.aspx?itemnumber=1). For effective dose calculation, the web application requires the user to input the type of nuclear medicine exam, injected activity (mCi or MBq), patient model (gender-average 1-, 5-, 10-, 15-year-old, adult, and an early pregnant woman), and dosimetry table (ICRP Publication 128 or RADAR 2017). The program uses two sets of absorbed dose conversion coefficients: ICRP Publication 128 based on the ORNL stylized phantoms and RADAR 2017. The web program does not provide organ-level dosimetry. The user interface of the web program is shown in Fig. 2D.
5. MIRDcalc
The website MIRDsoft (https://mirdsoft.org) was created to host free software applications designed and developed to support the medical radiation dose community. The MIRDcalc internal dosimetry software [85] was developed for calculating organ-level doses for radiopharmaceuticals. The program includes the SAF library calculated from the ICRP pediatric and adult voxel phantoms and decay data for 333 radioisotopes obtained from ICRP Publication 107. A total of 81 source and 43 target regions and five spherical tumor models were included in the SAF library. MIRDcalc was developed using Windows Visual Basic for Applications in Excel, so the program requires Microsoft Excel to be pre-installed. The program can be obtained from their website ( https://mirdsoft.org/ ) after creating an account. Fig. 2E shows the graphical user interface of MIRDcalc version 1.0.
6. OpenDose
OpenDose collaboration is intended to bring together the resources and expertise of research teams involved in nuclear medicine dosimetry [86]. The collaboration has been participated in by 18 international research teams from 13 countries worldwide. The website (https://opendose.org) currently provides decay data extracted from ICRP Publication 107, characteristics of the ICRP adult male and female voxel phantoms [50], SAFs from the ICRP adult voxel phantoms [56], and S values derived from the SAFs and the decay data for the ICRP adult male and female phantoms. The web application displays a bar graph and table of S values when a user inputs the phantom, source region, and radioisotope. Organ-level dose calculation is still in progress. Fig. 2F shows the web application of S value calculations for the selected patient models, source regions, and radioisotope.
7. NCINM
United States National Cancer Institute dosimetry system for Nuclear Medicine (NCINM) is an organ-level dose and effective dose calculator for patients undergoing nuclear medicine procedures. NCINM version 1.0 [72] included a library of S values derived from the 12 UF/NCI pediatric and adult hybrid phantoms and the decay data for 299 radiopharmaceuticals extracted from ICRP Publication 107, frequently used in diagnostic nuclear medicine procedures. The S values are available for 68 source regions and 55 target regions, which are listed in ICRP Publication 110 [50]. For the thin radiosensitive layers of the alimentary tract, the SAFs in ICRP Publication 133 were adopted. A user must manually input biokinetic data. The program was recently upgraded to NCINM version 2.0 [61] by implementing the S value library calculated from the 12 ICRP pediatric [59] and adult [50] voxel phantoms. The biokinetic data for 105 radiopharmaceuticals were extracted from ICRP Publication 53 [13], 80 [17], and 106 [18]. The original data derived for the anatomical definition of the stylized phantoms were modified to match the source definition of the ICRP reference voxel phantoms and then implemented into NCINM 2.0. Users can select one of the 105 radiopharmaceuticals to automatically populate TIAC to different source regions. The program was developed using a multi-platform GUI-based language, Xojo (Xojo Inc.), so the identical standalone program can operate on multiple operating systems, including Windows, Mac, and Linux. The program can be obtained through the United States National Cancer Institute website (https://dceg.cancer.gov/tools/radiation-dosimetry-tools) after submitting a software transfer agreement. Fig. 2G shows the graphical user interface of NCINM.
Research Gaps
In the past decades, there have been substantial advances in biokinetic data, libraries of internal dosimetry quantities, and computer programs for organ-level dose calculations for nuclear medicine procedures. However, there are still several gaps in the status of research in the areas of biokinetic models and energy transfer libraries.
The biokinetic data established to date are based on the anatomical system of the stylized phantoms, which cannot be directly translated to those of the modern voxel or hybrid phantoms. For example, the biokinetic data in ICRP Publications are provided for the upper and lower large intestines, which are the definition of the colon in the ORNL stylized phantoms. However, the colon in the ICRP voxel phantoms is divided into the left, right, and rectosigmoid colon. Andersson et al. [83] suggested that 71% of the TIAC in the lower large intestine can be assigned to the right colon, 29% of the TIAC in the upper large intestine and 56% of the TIAC in the lower large intestine assigned to the left colon, and 44% of the TIAC in the lower large intestine assigned to the rectosigmoid. Another example of the mismatch between the existing biokinetic data and the anatomy of the modern phantoms is the biokinetic models for the walled organs. The TIACs in whole organs are provided in existing ICRP Publications for the walled organs such as the gallbladder, heart, small intestine, stomach, and urinary bladder. However, these organs are separated into the wall and contents in the modern voxel or hybrid phantoms. One approach to resolve the issue is to separate the TIACs from the whole organs into the wall and contents by mass weighting. To avoid potential confusion, consistent efforts must be made to translate the existing biokinetic data into the detailed anatomical system of modern computational human phantoms.
The accuracy of SAFs has been substantially improved by the introduction of the modern voxel or hybrid computational phantoms, but the current SAFs still pose two limitations. First, the SAFs for the thin radiosensitive layers in the alimentary and gastrointestinal tracts are still based on the stylistic models in the ICRP Publications 66 [57] and 100 [58], respectively. The simplified model-based SAFs still have been adopted in the latest ICRP Publication 133 [56] on the adult SAFs and ongoing publication on the pediatric SAFs, which recently finished public consultation. The values can be updated by using more realistic organ models in the mesh based ICRP phantoms [76]. Second, the current SAFs are based on the reference body sizes. However, it is known that there is a substantial variation in body weight even in the same age group. There are two kinds of fat in human anatomy: visceral fat, found deep within the abdominal cavity, and subcutaneous fat, found just under the skin. Subcutaneous fat may not impact internal dosimetry, but visceral fat may increase the interorgan distances so decrease crossfire energy transfer. The dosimetric impact of body size has been actively investigated in other medical radiation procedures such as computed tomography [90–92] and fluoroscopy [93, 94]. However, little is known about the impact of body size on internal dosimetry to date.
It should be noted that the nomenclature and calculation methods for effective doses have changed over the past years. Effective dose equivalent derived using tissue weighting factors defined in ICRP Publication 26 [62] is reported in ICRP Publication 53. However, the term was changed to effective dose with new tissue weighting factors defined in ICRP Publication 60 [95] and adopted in the following addenda: ICRP Publications 62, 80, and 106. Tissue weighting factors were revised again in ICRP Publication 103, but the effective dose in the third and fourth addenda published after Publication 103 was still calculated using those in ICRP Publication 60. The recent revision of SAFs using the ICRP reference pediatric and adult voxel phantoms requires recalculation of absorbed and effective dose coefficients published in ICRP Publications 53, 62, 80, and 128. Effective dose coefficients should be calculated using the latest tissue weighting factors in ICRP Publication 103 [49].
Conclusion
In the current review article, model-based internal dosimetry methods were reviewed focusing on MIRD formalism, biokinetic data, human anatomy models (stylized, voxel, and hybrid computational human phantoms), and energy spectrum data of radionuclides. Key results from many articles on nuclear medicine dosimetry and comparisons of dosimetry quantities among different types of human anatomy models were summarized. Key characteristics of seven model-based dose calculation tools were tabulated and discussed including dose quantities, computational human phantoms used for dose calculations, decay data for radionuclides, biokinetic data, and user interface. Future research should focus on updating biokinetic data, revising energy transfer quantities for alimentary and gastrointestinal tracts, accounting for body size in nuclear medicine dosimetry, and recalculating dose coefficients based on the latest biokinetic and energy transfer data.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was funded by the intramural research program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.