Evaluation of the Efficacy of Zoledronic Acid and Amifostine on Radiation-induced Bone Loss in Mice
Article information
Abstract
Background
This study investigated the effects of zoledronic acid (ZA) on radiation-induced bone loss in C3H/HeN mice.
Materials and Methods
C3H/HeN mice were divided into sham control and three irradiated groups (3 Gy, gamma ray). The irradiated mice were treated for 12 weeks with vehicle, amifostine (intraperitoneal injection), or ZA (subcutaneous injection). Grip strength, uterus weight, and serum alkaline phosphatase (ALP), and tartrate-resistant acid phosphatase (TRAP) levels were measured. Tibiae were analyzed using micro-computed tomography.
Results and Discussion
Treatment of ZA (100 μg·kg−1·week−1) significantly preserved trabecular bone volume, trabecular thickness, trabecular number, trabecular separation, bone mineral density of proximal tibia metaphysic, and cortical bone volume, but did not alter the uterus weight of the mice. The administration of ZA for 12 weeks lowered serum ALP and TRAP levels in irradiated mice, suggesting that ZA can reduce the bone turnover rate in mice. No differences were apparent between the amifostine-treated group and the irradiation control group.
Conclusion
The results indicate that ZA can prevent radiation-induced bone loss in mice.
Introduction
Developments in cancer therapies and diagnostic techniques have improved the long-term survival of cancer patients [1]. There has been a corresponding increase in the prevalence of specific and chronic side effects of cancer treatment among survivors. Certain cancer treatments, such as radiotherapy, often harm normal tissue as well as the specifically targeted cancer cells [2]. The underlying mechanism of these adverse effects and the prognoses associated with them deserve greater research attention.
High doses of radiation induce bone loss. Irradiation of bone principally causes atrophy accompanied by compromised function and structure of the bone tissue, but does not result in alterations of the bone size. Changes in vasculature, composition of bone matrix, and cellular components are putative etiologies of irradiation-induced damage in bone [3]. Irradiation of bone has also been associated with fractures [4]. Osteoporosis is a public health concern with a heavy financial burden on both society and patients [5]. Drugs for osteoporosis aim to augment the bone regeneration process as opposed to the bone resorption process. Inhibitors of bone resorption include estrogen, calcitonin, bisphosphonates, calcium, vitamin D, and raloxifene. Accelerators of bone formation include fluorides and anabolic steroids [6].
Zoledronic acid (ZA), a type of bisphosphonate, is a known prophylactic agent against osteoporosis. It is used in the clinical setting to treat patients whose cancer has already metastasized to the bone. By reducing the activity of osteoclasts, ZA prevents metastasis of cancer cells to the bone and alleviates pathologic fractures [7]. Zoledronic acid is a third generation bisphosphonate that has anti-catabolic activity, which is the most effective way to inhibit bone resorption. A single infusion of 5 mg of ZA given annually was shown to decrease the rate of osteoporotic fractures. By the third year of medication in that study, and compared to the control group, the ZA treatment group had 70% less vertebral fractures, 41% less zygomatic fractures, and 25% less non vertebral fractures [8, 9]. Despite evidence for anti-osteoporotic effects of ZA, not many studies suggest the protective effect of ZA in radiation-induced bone loss. In this study, we investigated the efficacy of two agents to scavenge for radiation-induced free radicals in irradiated mice: amifostine, a radioprotective agent [10]; and ZA, an anti-osteoporotic agent.
Materials and Methods
1. Animals and irradiation
We irradiated mature C3H/HeN mice (OrientBio, Seongnam, Korea), which were determined to have the optimal trabecular bone volume for this study at around 9 weeks. After acclimatization under standard conditions (temperature of 22±2°C; relative humidity of 50±10%; 12 hours of light between 8 am and 8 pm; and luminance of 200–300 lux), 3 mice were placed in each polycarbonate box (2 boxes per treatment group), and all mice were provided standard laboratory rodent chow (Samyang Food, Wonju, Korea) and filtered water ad libitum. All animal experiments adhered to the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources and were approved by the Institutional Animal Care and Use Committee at Chonnam University (Approval number: CNU IACUC-YB-2015-3). Using a laboratory irradiator (Gamma-cell Elan 3000, Nordion International, Ottawa, Canada), we performed whole-body irradiation based on the protocol optimized in our preliminary study [11]. Mice were irradiated simultaneously and received a total dose of 3 Gy of 137Cs (a dose rate of 3.2 Gy·min−1). We measured the changes in bone parameters 12 weeks post irradiation.
2. Treatment groups and chemical treatment
We divided the mice into 4 treatment groups each consisting of 6 mice: the sham control group that received no intervention at all; the irradiation control group; the amifostine-treated irradiated group (Sigma chemicals, St. Louis, MO); and the ZA-treated irradiated group (Novartis Pharma Stein AG, Stein, Switzerland). In the amifostine-treated irradiated group, mice were treated with a single intraperitoneal injection of 214 μg·kg−1 of amifostine 30 minutes before irradiation [12]. In the ZA-treated irradiated group, mice were pre-treated with a weekly subcutaneous injection of 100 μg·kg−1 of ZA for 3 days before irradiation, following the protocol used in a study that investigated the effect of concurrent administration of ZA and irradiation in mice with ovariectomy-induced bone loss [13].
3. Measurement of grip strength, study endpoint, and serum analyses
We evaluated the general symptoms at regular intervals during the treatment period, and the mice were weighed before initiation of treatment and at the time of killing. To measure grip strength in mice, we used a tensile-force measurement device (Iwoo-G-500, Iwoo Scientific Corp, Seoul, Korea). First, we let the mice take hold of the measurement pole with its forefeet and calibrated the device to zero. Then we pulled the tail gradually away from the body and measured the point at which the forefeet lost grip of the measurement pole, regarding this as the maximal grip strength. A total of 3 measurements were taken for grip-strength, and the average of these repeats was calculated. To measure tibial strength, we performed a triple flexion response. We placed the weight at the center of two points that were 14 mm apart, and marked as the loading point a region that was 7 mm distal from the weight; the tibia was positioned accordingly. Taking the medial-lateral axis as our standard, we induced flexion. The load-deflection curve was measured using the servo-hydraulic material testing machine (Instron Ltd, Buckinghamshire, UK) and the x-ray recorder (Hewlett Packard 7090A, Palo Alto, CA) at a crosshead speed of 1 mm·sec−1. Maximum weight bearing reflects the maximum stress on the tibia, experienced, for instance, when a load is placed on a fractured site. Mice were humanely killed at 12 weeks post irradiation and mice organs were weighed concurrently. In addition to gross findings of each organ, we carried out a histopathological examination if any abnormalities were detected. The mass of the two main organs observed—the tibia and the uterus—were measured. The left tibia was stored in 70% ethanol until its bone mineral density was measured and computed tomography scans were taken. At the time of necropsy, we collected blood samples from the caudal vena cava and separated the serum. Using a blood chemistry analyzer (FDC 3500s, Fuji film, Tokyo, Japan), we measured the serum concentration of alkaline phosphatase (ALP), a cytochemical marker of osteoblast activity. Using an enzyme-linked immunosorbent assay (ELISA) measurement kit (TRACP-5b ELISA Kit, Antibodies-online, Aachen, Germany), we measured the serum concentration of tartrate resistant acid phosphatase (TRAP), a cytochemical marker of osteoclast activity.
4. Micro-computed tomography (micro-CT)
We used micro-computed tomography (micro-CT) to perform topography of the samples (Skyscan 1172; Skyscan, Kontich, Belgium). First, using a cone beam x-ray source, we irradiated the samples under 50 kV and 200 μA (spatial resolution was set to 17 μm). Samples were irradiated every 1.2 seconds and rotated 0.4° each time. Images were taken as the beam passed through the focus spot of the sample, and the information was passed and transferred onto the charge-coupled devices (CCD). Using NRecon software (Skyscan, Kontich, Belgium), the obtained images were reconstructed into 150 two-dimensional (2-D) cross-sections, where the distance between each cross-section was 80 μm. The 2-D images of the bone were used to analyze the microarchitecture of the tibia using CTan software (Skyscan, Kontich, Belgium). Trabecular bone was regarded as the area 1.5 mm below the growth plate. Cortical bone was measured from 1.0 mm below the area of trabecular bone loss. On the reconstructed 2-D images, we discriminated between the cortical bone and the trabecular bone, and marked the region of interest. We measured the following quantitative parameters of the cortical bone and trabecular bone: bone volume/tissue volume (BV/TV); trabecular thickness (Tb.Th); trabecular separation (Tb.Sp); trabecular number (Tb.N); structural parameters, such as structure model index (SMI); and trabecular bone pattern factor (Tb.Pf). We marked another region of interest that included a portion of the trabecular bone to use as our standard in the comparative analysis. To measure bone mineral density (BMD), we applied the Hounsfield units (HU) of the sample we wanted to measure into the equation after we had measured HU for 0.25 g·cm−3 and 0.75 g·cm−3 of hydroxyapatite standard phantom. We also calculated bone volume (BV) and polar moment of inertia (pMOI).
5. Statistical analysis
All statistical analyses were performed using Graph PAD In Plot computer program (GPIP; Graph PAD Software, San Diego, CA). We used a two-tailed Student t test to assess the statistical significance between groups. We summarized the data into mean±SD. Statistical significance was set at p-value of less than 0.05 for all comparisons.
Results and Discussion
There are numerous reports on the favorable effects of ZA for osteoporosis, but those specifically describing an anti-osteoporotic effect of ZA following radiation treatment are few. In this study, we investigated various parameters of trabecular bone, of cortical bone, and of bone mineral density in irradiated mice and assessed the efficacy of amifostine and ZA to prevent radiation-induced bone loss.
We found that grip strength, body mass, and uterine mass did not show a significant difference among treatment groups (Figure 1). The observed changes in serum cytochemical markers are summarized in Figure 2. We found that serum ALP was significantly reduced (P<0.05) in amifostine-treated and in ZA-treated irradiated mice in relation to the irradiation control mice. Serum TRAP was significantly lower in ZA-treated irradiated mice than in the irradiation control mice. The representative micro-CT tibial images from each treatment group are displayed in Figure 3. Our analysis of the micro-CT tibial images shows that trabecular bone volume was 33% lower in the irradiation control group than in the sham control group. Further, BV/TV was 5.9-fold greater in ZA-treated mice than in irradiation control group; these patterns were similar for Tb.Th and Tb.N. We found that Tb.Sp was 71% smaller in the ZA-treated irradiated group compared to the irradiation control group, and that BMD of the trabecular bone in the ZA-treated irradiated group was 61-fold greater than that of the irradiation control group (Figure 4). The BV was 72% larger in ZA-treated irradiated mice than in mice that did not receive ZA. We did not observe an anti-osteoporotic effect for amifostine treatment.
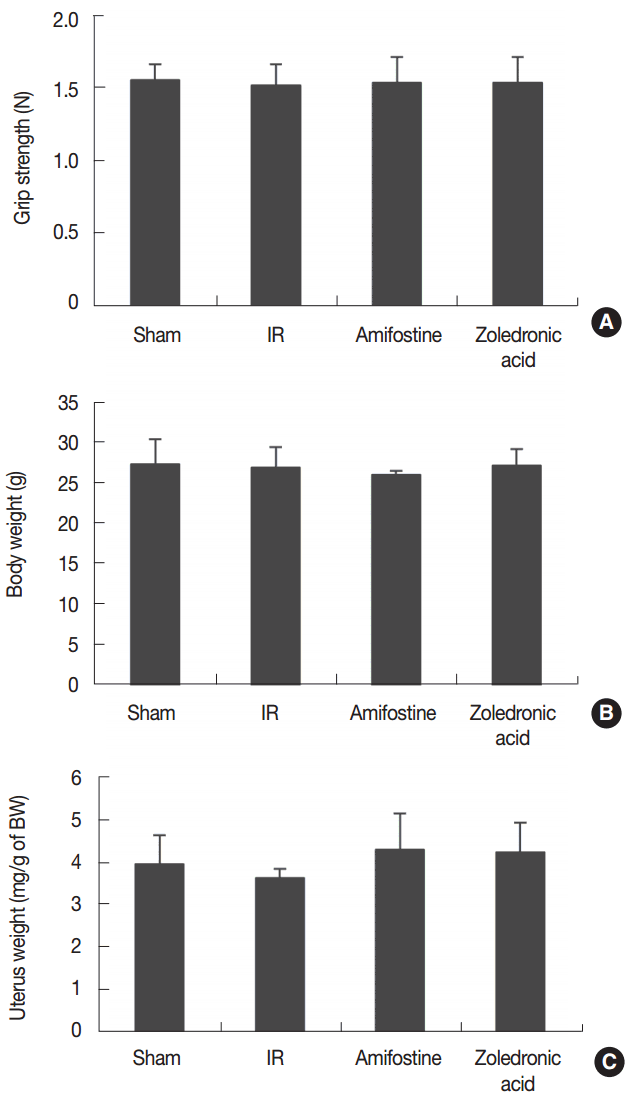
Effect of amifostine and zoledronic acid on grip strength (A), body weight (B) and uterine weight (C) in irradiated (IR, 3 Gy) mice. Amifostine was given (214 μg·kg−1) intraperitoneally at 30 minutes before irradiation. Zoledronic acid was given (100 μg·kg−1·week−1) subcutaneously for 12 weeks after irradiation. Data are expressed as means±SD (n=6).
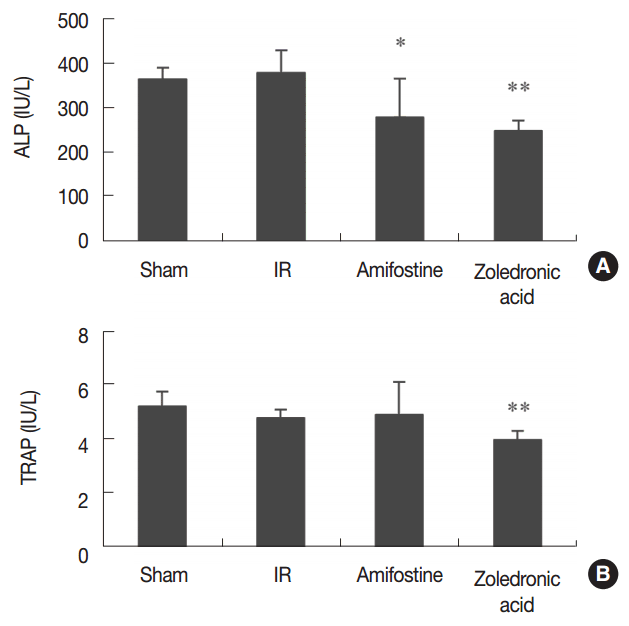
Effect of amifostine and zoledronic acid on serum alkaline phosphatase (ALP, A) and tartrate-resistant acid phosphatase (TRAP, B) in irradiated mice. Data are expressed as means±SD (n=6). *P<0.05, **P<0.01 vs. irradiation control group at corresponding parameters.

Representative micro-CT three-dimensional images of trabecular architecture of tibia in (A) sham control, (B) irradiation control and (C) zoledronic acid-treated irradiated mouse.
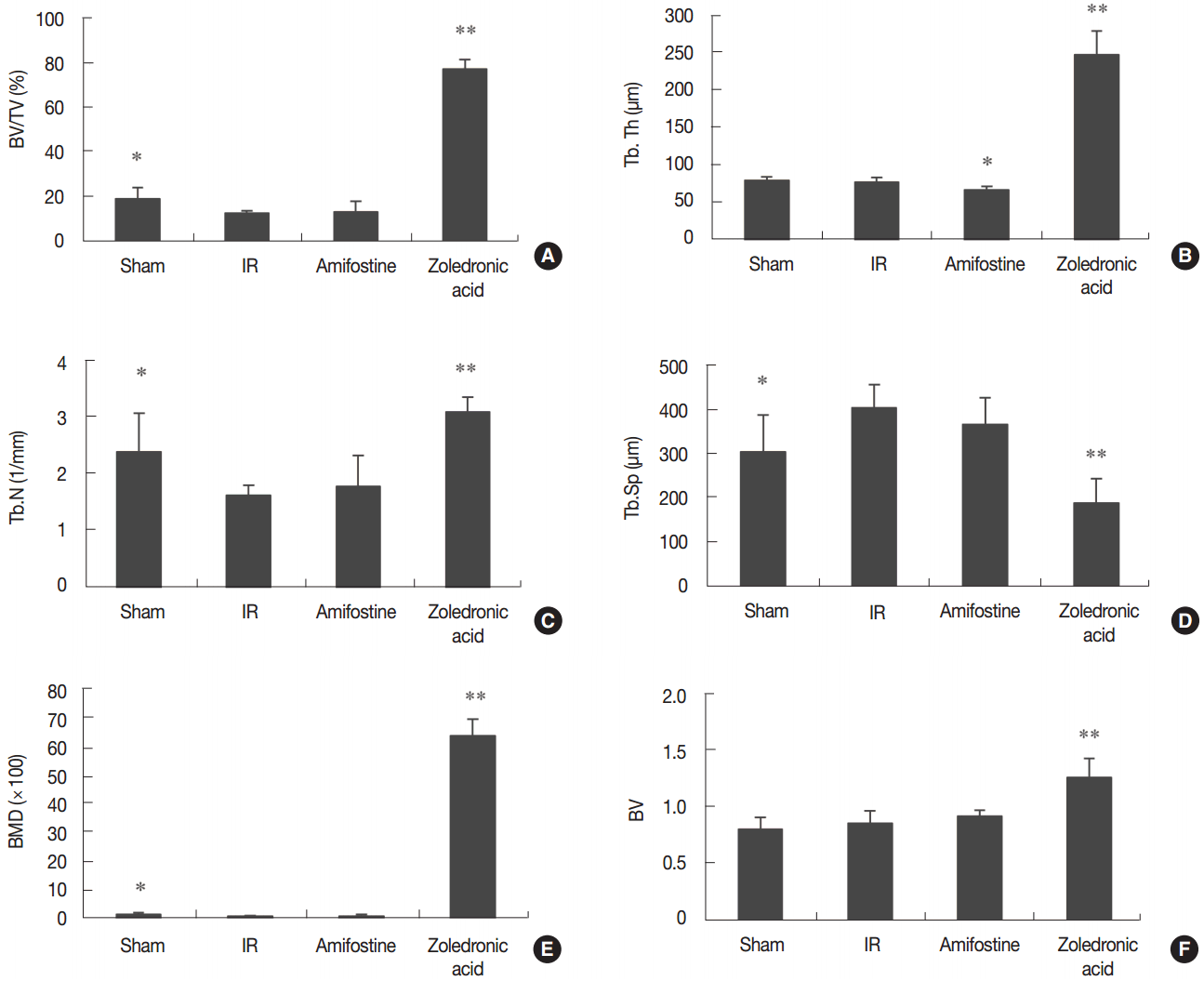
Effect of amifostine and zoledronic acid on trabecular (A–E) and cortical (F) bone properties of tibia in irradiated mice. BV/TV: bone volume density (A); Tb.Th: trabecular thickness (B); Tb.N: trabecular number (C); Tb.Sp: trabecular separation (D); BMD: trabecular volumetric mineral density (E); BV: cortical bone volume (F). Data are expressed as means±SD (n=6). *P<0.05, **P<0.01 vs. IR group at corresponding parameters.
Fractures are one of the chronic side effects of radiotherapy. Although the etiology of irradiation-induced fractures is not understood, it is known that ionizing radiation leads to a chronic reaction that causes damage to both intramedullary and cortical osteoblasts and to the vasculature; as a result, bone mineral density decreases and the risk of fractures increases [14, 15]. Data from in vivo and in vitro studies showed that damage and depletion of osteoblasts upon radiation exposure contribute to various aspects of dysfunctional bone remodeling, particularly the abnormal proliferation and differentiation of osteoblasts, uncontrolled cell cycle arrest, reduced collagen production, and increased sensitivity to apoptotic factors [16, 17]. The correlation between a damaged bone and a compromised circulatory system has not yet been supported with empirical evidence [15], but the correlation between chronic bone atrophy and irradiation has been supported through clinical and animal studies [18, 19].
Although studies have shown the effect of radiotherapy on the reduction of osteoclast numbers and on the imbalance of bone remodeling, the effect of radiotherapy on osteoclast activity remains unclear [20, 21]. A few studies have suggested that the early stimulus of bone resorption after irradiation triggers irradiation-induced bone loss [22]. A high serum level of bone regeneration factors is reflective of this heightened propensity for bone regeneration [23], and is associated with rapid bone resorption during osteoporosis. Increased bone regeneration factors and decreased BMD factors correspond to an especially heightened risk for fractures [24]. Profiles of bone remodeling factors are used to assess the extent of bone loss and also to assess the efficacy of various anti-osteoporotic therapies [23]. As an effective inhibitor of bone resorption, infusion of bisphosphonate ZA is regarded as one of the principal prophylactic measures against osteoporosis. In this study, we confirmed the protective effects of ZA against bone loss after irradiation in mice. We found that, compared to the irradiation control group, the ZA-treated irradiated group showed lower ALP and TRAP serum levels. These results imply that the mechanism of the radioprotective effects of ZA against bone loss may occur by counteracting the irradiation-induced imbalance of bone remodeling.
Mechanical studies show a correlation among grip strength, bone flexion strength, and BMD [25], but we could not corroborate these findings in our study. We measured uterine mass in mice because it was previously hypothesized that radiation-induced uterine dysfunction and resulting hormonal changes, especially levels in estrogen, may promote bone loss. However, none of the treatment groups showed a statistically significant difference in terms of uterine mass, implying that bone loss is not secondary to uterine dysfunction.
Our experimental data support the protective role of ZA against bone loss in irradiated mice. Based on the findings of this study, development of ZA-based treatments is anticipated to address bone loss after radiotherapy. Prospective dose escalation studies are required to determine the appropriate dosage of ZA.
Conclusion
In this study, we investigated the efficacy of ZA to prevent bone loss in irradiated mice. Our micro-CT analysis of the tibiae showed a significant reduction in bone loss among ZA-treated irradiated mice. ZA-treatment in irradiated mice was associated with an improvement in several bone parameters such as trabecular bone volume, trabecular thickness, trabecular number, trabecular separation, trabecular bone mineral density, and cortical bone volume. The lower blood ALP and TRAP levels in ZA-treated irradiated mice compared to irradiation control mice, suggests the underlying mechanistic actions of ZA promotes bone regeneration leading to its anti-osteoporotic effects.
Acknowledgments
This study was conducted with support provided by Chonnam National University in 2014.