AbstractBackgroundAs breast tissue expanders consist of metallic materials in the needle guard and ferromagnetic injection port, irradiation can produce radioactivation.
Materials and MethodsA CPX4 (Mentor Worldwide LLD) breast tissue expander was exposed using the Versa HD (Elekta) linear accelerator. Two photon energies of 6 and 10 MV-flattening filter free (FFF) beams with 5,000 monitor units (MU) were irradiated to identify the types of radiation. Furthermore, 300 MU with 10 MV-FFF beam was exposed to the CPX4 breast tissue expander by varying the machine dose rates (MDRs) 600, 1,200, and 2,200 MU/min. To assess the instantaneous dose rates (IDRs) solely from the CPX4, a tissue expander was placed outside the treatment room after beam irradiation, and a portable radioisotope identification device was used to identify the types of radiation and measure IDR.
Results and DiscussionAfter 5,000 MU delivery to the CPX4 breast tissue expander, the energy spectrum whose peak energy of 511 keV was found with 10 MV-FFF, while there was no resultant one with 6 MV-FFF. The time of each measurement was 1 minute, and the mean IDRs from the 10 MV-FFF were 0.407, 0.231, and 0.180 μSv/hr for the three successive measurements. Following 10 MV-FFF beam irradiation with 300 MU indicated around the background level from the first measurement regardless of MDRs.
ConclusionAs each institute room entry time protocol varies according to the working hours and occupational doses, we suggest an addition of 1 minute from the institutes’ own room entry time protocol in patients with CPX4 tissue expander and the case of radiotherapy vaults equipped with a maximum energy of 10 MV photon beams.
IntroductionFor most women who undergo total mastectomy, breast reconstruction is a preferable option with the advantages of relative simplicity, low morbidity, and cosmetic outcomes [1, 2]. The most widely used reconstruction process involves two stages: temporary tissue expansion and permanent implantation [3], accounting for approximately 61% of all breast reconstruction procedures in the United States [4]. Tissue expansion is intended to reserve sufficient space by stretching the skin and muscles when excessive resections are made without sufficient breast tissue [5]. The breast tissue expander is a balloon-like inflatable device with a silicone outer shell and a superficial port [6]. Once a tissue expander is inserted during the surgical stage, saline is injected on a regular basis [6]. Once they attain the desired shape and size, the tissue expander is removed and replaced with permanent saline or silicone breast prostheses [6].
Nowadays, breast radiation therapy is usually administered by an intensity modulated radiation therapy technique using low-energy photon beams (usually around 6 MV) because this enables a substantial reduction in toxicity while providing sufficient target coverage [7, 8]. However, it has been reported that patients who received postmastectomy radiotherapy (RT) in the presence of tissue expanders tend to be less effective and have an increased risk of complications than those who received permanent implants [9] or autologous tissue reconstruction [10]. One reason for this is the perturbation of the dose distribution, and this is mainly because the extremely high-density of the metallic port of the tissue expander causes a discrepancy between the calculated and measured dose distribution [11–15]. There are metallic materials in the needle guard and ferromagnetic injection port of the CPX4 breast tissue expander (Mentor Worldwide LLD), consisting of 316 L stainless steel and neodymium, respectively. Although it varies with the Hounsfield unit modeling coverage of the high-density materials in the treatment planning system, it has been reported that the amount of dose mismatch can be mitigated using higher photon (>10 MV) energies along with a build-up compensating bolus [15–17]. Furthermore, metal artifacts in computed tomography images caused by metallic components often hinder the correct segmentation of the breast and adjacent tissues [14, 18]. As distinct from the above-mentioned issues, there is a possibility of temporary radioactivation (TRA) induced from the tissue expander with radiation exposures. Radioactive nuclides are generated when high-energy photon beams irradiate high-density materials owing to photonuclear reactions [19]. Although many studies have focused on the radioactivation of high-atomic number materials in the RT vault [20–23], to the best of our knowledge, no previous studies have demonstrated the TRA of the tissue expander.
In this study, we utilized a portable radioisotope identification device (RIID) to identify the types of radiation and measured the instantaneous dose rates (IDRs) when the CPX4 breast tissue expander was irradiated with respect to the machine dose rate (MDR) and incident energy. We also identified the duration at which the measured dose was below a safe level.
Materials and Methods2. Experiment DesignRecently, breast RT is widely conducted with hypofractionation of which a daily dose of 2.7 Gy, although it could vary according to the pathological stage of the tumor [24, 25]. To cover a common daily dose, we designed to deliver 3 Gy to the surface of the breast tissue expander. Two photon energies of 6 and 10 MV-flattening filter free (FFF) beams from the Versa HD linear accelerator (LINAC; Elekta) were used in this study. We used an FFF beam rather than a beam with flattening filter to assess the IDR according to the MDR.
The experimental setup is depicted in Fig. 2. A 5-cm solid water phantom was placed on the table-top for backscatter and slanted polystyrene to adjust the tilted geometry of the CPX4 breast tissue expander. Slanted polystyrene was used to make the beam incidence perpendicular to the tissue expander, thus delivering a uniform dose. Above a tissue expander, the energy-dependent thickness of the commercial bolus (1.5 cm for 6 MV-FFF and 2.5 cm for 10 MV-FFF) was also placed for build-up. The source-to-surface distance was set to 100 cm, and the field size was set to 10×10 cm2.
To demonstrate the types of radiation according to the energy, 5,000 monitor units (MU) were delivered with 6 and 10 MV-FFF. To demonstrate the TRA according to the MDR, 300 MU with 10 MV-FFF beams were irradiated by varying the MDRs of the 600, 1,200, and 2,200 MU/min.
3. Measurement of RadioactivationA RIIDEye X-GN detector (Model 42508/85; Thermo Fisher Scientific Messtechnik GmbH) was used to detect the presence of any types of radiation. The detector was calibrated using the test sources 137Cs, 226Ra, and 252Cf, and each peak energy was all within the tolerance limits (±2% for 137Cs and 226Ra, and ±20% for 252Cf). The RIIDEye X-GN consisted of a 5.08 cm×5.08 cm NaI gamma detector with a typical resolution of 7.5% at 662 keV and detectable gamma energy ranging from 25 keV to 3 MeV. Furthermore, an 18 mm×34 mm crystal was internally separated from the gamma detector, which enabled the detection of neutron count rates up to 2,000 counts per second. Data from all measurements were stored in Ansi N42 format. When all experiments were completed, RIIDView software version 1.0 (Thermo Fisher Scientific Messtechnik GmbH) was used to analyze IDR, measured energy spectrum, and isotopes estimated using a 256 channel-based quadratic compression conversion algorithm. Additionally, another software, InterSpec version 1.0.11 (National Technology & Engineering Solutions of Sandia LLC), was used because the RIIDView software does not support exporting raw spectrum data. This software supported the export of each spectrum, thus facilitating direct comparisons of the energy spectrums across measurements. Based on the spectrum, the software suggests the most well-matched nuclide by searching for it in a predefined nuclide database.
Prior to beam irradiation, the RIIDEye X-GN underwent background correction. After beam irradiation, a tissue expander was placed outside the treatment room to assess the IDRs solely from the CPX4 breast tissue expander not from the LINAC head, and the TRA was measured for 60 seconds. If any nuclides were detected or the IDR was measured to be higher than the background level, the measurement continued until there were no nuclides or the IDR decreased to the background level. All measurements were repeated twice to demonstrate the statistical uncertainties.
Results and DiscussionIn general places far away from the treatment room, RIIDEye X-GN showed a mean IDR of 0.181 μSv/hr, which was set as the background level. As shown in Fig. 3, the IDRs after delivering 5,000 MU with 10 MV-FFF were 0.407±0.011, 0.231±0.001, and 0.180±0.004 μSv/hr for three sequential measurements. The IDR reached the background level except for the first measurement. Fig. 4 presents the first measured energy spectrum after 5,000 MU delivered for 10 MV-FFF showing negligible counts within 3 minutes. There was notable energy spectrum whose peak energy of 511 keV was found with 10 MV-FFF, while there was no obvious one with 6 MV-FFF. No neutron fragments were detected in either case.
The IDR measurements with 300 MU according to MDR are presented in Fig. 5, and are shown around the background level from the first measurement. Furthermore, it was not significantly affected by the MDR (600, 1,200, and 2,200 MU/min). No neutron fragments were detected in either experiment. Although a peak energy of 511 keV was detected when 300 MU was delivered to the CPX4 tissue expander, the corresponding counts were negligible, as shown in Fig. 6.
It is a matter of course that the measured IDRs from a tissue expander was much lower than that of LINAC head. Previous research has demonstrated that the IDR was 4.14 μSv/hr immediately after 1,000 MU delivery with 15 MV, and decreased to 0.65 μSv/hr after 5 minutes [26]. Another study showed that the measured dose rate was 0.702 μSv/hr right after 500 MU delivery with 10 MV, and decreased to 0.406 μSv/hr after 1 minute [27]. Although it could vary depending on the LINAC products and incident energy, it is definitely greater than those from tissue expanders. The LINAC head-generated neutrons are mainly due to (γ, n) reactions, and the produced neutron might cause nuclear reactions itself and the tissue expanders [28, 29]. Although the half-life of a single neutron in air is microseconds, the radioactivity remains and should be considered its accumulation [30]. Even though the specific activation process is unknown because of its multicompounds of tissue expanders, authors could assume that the majority of activation was induced from high-density materials, i.e., stainless steel and neodymium magnet, whose physical densities are 8.1 and 7.01 g/cm3, respectively.
Furthermore, the mean occupational radiation doses for medical fields in South Korea and those from the United Nations Scientific Committee on the Effects of Atomic Radiation annual report in 2020 were 0.38 and 0.57 mSv, respectively, which could be regarded as keeping well as low as reasonably achievable practice when applying occupational dose limit [31–33]. Referring to both IDRs from LINAC head and national occupational radiation dose statistics, tissue expander-induced IDRs may not be a great concern because even slightly higher IDRs of 0.392 μSv/hr was caused by extremely high MU delivery. Moreover, the prescription greater than 10 Gy could not be a real clinical situation. Even if hypofractionated RT to breast and supraclavicular lymph nodes often employs monitor units greater than 1,000 MU [34], the MU should not be evaluated alone because the delivered dose was determined not only by the amount of MU but also by the other beam parameters such as field size and source-to-axis distance [35]. Despite the 5,000 MU delivery with a 10 MV-FFF photon beam with a field aperture of 10 cm×10 cm, the IDR was reduced to the background level after 1 minute.
The optimal room entry time was discussed from the literatures and it varied a lot [30, 36]. As they varied upon the number of patients and the proportion of treatment sites related to the used energy, each institute established the room entry time protocols considering both working hours and occupational equivalent doses.
Figs. 4, 6 indicate that while 10 MV irradiation to the CPX4 tissue expander could generate an energy spectrum whose peak energy was around 511 keV, this corresponded to the background level within 1 minute. In this regard and considering that the experiment was performed outside the treatment room, an additional delay time of 1 minute from institutes’ own protocols after treatment of patients with CPX4 tissue expander could guarantee that there is little harm to RT staff, and it was not influenced by the amount of irradiation dose. There was no notable energy peak with 6 MV-FFF regardless of the dose, indicating that no additional delay time due to the use of tissue expanders is necessary for the RT staff.
This study had several limitations. First, other kinds of tissue expanders exist. Tissue expander products from the same company (Mentor Worldwide LLD) vary according to size, shape, and product type. We expect that in-depth experiments, including other kinds of tissue expanders with different sizes, shapes, and constituent materials, could provide insights into clinical protocol establishment or radiation safety perspectives. Second, the measurements could not be performed with 15 MV in this study because of the absence of 15 MV with this LINAC. While the use of a 15 MV photon beam is known to reduce dose heterogeneity and improve dose coverage in patients with tissue expanders [11, 16], it is also known that photoneutron generation and radioactivation are more prevalent at higher photon energies. Any institute with LINACs equipped with 15 MV or higher should verify the types of isotope, amount of radioactivation, and their influence on energy and dose rate in advance treatments for patients with breast tissue expanders. Nonetheless, our study seems significant from a clinical protocol establishment or radiation safety perspective because the study is the first attempt to reveal the potential of TRA when performing breast RT in patients with tissue expanders.
ConclusionThis study demonstrated TRA of the CPX4 tissue expander when the expander was irradiated. While irradiation of 6 MV-FFF beams to the CPX4 tissue expander generated no resultant nuclides, those with 10 MV-FFF beams produced a spectrum with peak energies of 511 keV. However, experiments with 3 Gy delivery, exhibited IDRs of around background level within 1 minute, regardless of the MDR. In patients with CPX4 tissue expander and the case of RT vaults equipped with a maximum energy of 10 MV photon beams, the staffs are recommended to enter the treatment room by adding at least 1 minute from their own protocols because a room entry time protocol varies according to each institute.
AcknowledgementsThis study was supported by research grant from Biomedical Research Institute, Chung-Ang University Hospital (2022).
Authors would like to address a warm appreciation to RemTech Co., Ltd. to support a RIIDEye X-GN device.
NotesEthical Statement This article does not contain any studies with human participants or animals performed by any of the authors. Author Contribution Conceptualization: Lee H. Methodology: Lee H, Chun M. Data curation: Lee H, Yoo L. Formal analysis: Lee H, Oh DH, Chun M. Supervision: Lee H, Oh DH, Chun M. Funding acquisition: Chun M. Project administration: Chun M. Investigation: Lee H, Oh DH, Yoo L, Chun M. Visualization: Yoo L, Chun M. Validation: Lee H, Chun M. Writing - original draft: Lee H, Chun M. Writing - review and editing: Oh DH, Chun M. Approval of final manuscript: all authors. References1. McCarthy CM, Pusic AL, Disa JJ, McCormick BL, Montgomery LL, Cordeiro PG. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg. 2005;116(6):1642-1647.
2. Chen SA, Hiley C, Nickleach D, Petsuksiri J, Andic F, Riesterer O, et al. Breast reconstruction and post-mastectomy radiation practice. Radiat Oncol. 2013;8:45.
3. Maxwell D, Estes MM, Walcott JM, Canady JW, Hunter TD, Gache L, et al. Safety of CPX4 breast tissue expanders in primary reconstruction patients. Plast Reconstr Surg Glob Open. 2021;9(3):e3425.
4. American Society of Plastic Surgeons. 2020 Plastic surgery statistics report [Internet]. ASPS; 2021 [cited 2023 Jun 7]. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf
5. Bertozzi N, Pesce M, Santi P, Raposio E. Tissue expansion for breast reconstruction: methods and techniques. Ann Med Surg (Lond). 2017;21:34-44.
6. Wagh MS, Dixit V. Tissue expansion: concepts, techniques and unfavourable results. Indian J Plast Surg. 2013;46(2):333-348.
7. Buwenge M, Cammelli S, Ammendolia I, Tolento G, Zamagni A, Arcelli A, et al. Intensity modulated radiation therapy for breast cancer: current perspectives. Breast Cancer (Dove Med Press). 2017;9:121-126.
8. Fischbach M, Halg RA, Hartmann M, Besserer J, Gruber G, Schneider U. Measurement of skin and target dose in post-mastectomy radiotherapy using 4 and 6 MV photon beams. Radiat Oncol. 2013;8:270.
9. Nava MB, Pennati AE, Lozza L, Spano A, Zambetti M, Catanuto G. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128(2):353-359.
10. Chawla AK, Kachnic LA, Taghian AG, Niemierko A, Zapton DT, Powell SN. Radiotherapy and breast reconstruction: complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys. 2002;54(2):520-526.
11. Yoon J, Xie Y, Heins D, Zhang R. Modeling of the metallic port in breast tissue expanders for photon radiotherapy. J Appl Clin Med Phys. 2018;19(3):205-214.
12. Moni J, Graves-Ditman M, Cederna P, Griffith K, Krueger EA, Fraass BA, et al. Dosimetry around metallic ports in tissue expanders in patients receiving postmastectomy radiation therapy: an ex vivo evaluation. Med Dosim. 2004;29(1):49-54.
13. Chen SA, Ogunleye T, Dhabbaan A, Huang EH, Losken A, Gabram S, et al. Impact of internal metallic ports in temporary tissue expanders on postmastectomy radiation dose distribution. Int J Radiat Oncol Biol Phys. 2013;85(3):630-635.
14. Mizuno N, Takahashi H, Kawamori J, Nakamura N, Ogita M, Hatanaka S, et al. Determination of the appropriate physical density of internal metallic ports in temporary tissue expanders for the treatment planning of post-mastectomy radiation therapy. J Radiat Res. 2018;59(2):190-197.
15. Damast S, Beal K, Ballangrud A, Losasso TJ, Cordeiro PG, Disa JJ, et al. Do metallic ports in tissue expanders affect postmastectomy radiation delivery? Int J Radiat Oncol Biol Phys. 2006;66(1):305-310.
16. Chatzigiannis C, Lymperopoulou G, Sandilos P, Dardoufas C, Yakoumakis E, Georgiou E, et al. Dose perturbation in the radiotherapy of breast cancer patients implanted with the Magna-Site: a Monte Carlo study. J Appl Clin Med Phys. 2011;12(2):3295.
17. Sharabi A, Myers L, Dah S, Duhon M, Ford K, Zellars R, et al. Dosimetric impact and 3D nondeformable modeling of metallic breast expander ports during postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87(2 Suppl):S199.
18. Trombetta DM, Cardoso SC, Facure A, da Silva AX, da Rosa LA. Influence of the presence of tissue expanders on energy deposition for post-mastectomy radiotherapy. PLoS One. 2013;8(2):e55430.
19. Fischer HW, Tabot B, Poppe B. Comparison of activation products and induced dose rates in different high-energy medical linear accelerators. Health Phys. 2008;94(3):272-278.
20. International Atomic Energy Agency. Management of radioactive waste from the use of radionuclides in medicine. IAEA. 2000.
21. Kwon NH, Shin DO, Kim J, Yoo J, Park MS, Kim KB, et al. Current status of disposal and measurement analysis of radioactive components in linear accelerators in Korea. Nucl Eng Technol. 2022;54(2):507-513.
22. Lee DY, Kim JH, Park ET. Assessment of human exposure doses received by activation of medical linear accelerator components. J Instrum. 2017;12(8):P08022.
23. National Council on Radiation Protection and Measurements. NCRP report No 79: Neutron contamination from medical electron accelerators. NCRP. 1984.
24. Park HJ, Oh DH, Shin KH, Kim JH, Choi DH, Park W, et al. Patterns of practice in radiotherapy for breast cancer in Korea. J Breast Cancer. 2018;21(3):244-250.
25. Kim K, Chun M, Jin H, Jung W, Shin KH, Shin SS, et al. Inter-institutional variation in intensity-modulated radiotherapy for breast cancer in Korea (KROG 19-01). Anticancer Res. 2021;41(6):3145-3152.
26. Israngkul-Na-Ayuthaya I, Suriyapee S, Pengvanich P. Evaluation of equivalent dose from neutrons and activation products from a 15-MV X-ray LINAC. J Radiat Res. 2015;56(6):919-926.
27. Kwon NH, Jang YJ, Kim J, Kim KB, Yoo J, Ahn SH, et al. Measurements of neutron activation and dose rate induced by high-energy medical linear accelerator. Prog Med Phys. 2021;32(4):145-152.
28. Nedaie HA, Darestani H, Banaee N, Shagholi N, Mohammadi K, Shahvar A, et al. Neutron dose measurements of Varian and Elekta linacs by TLD600 and TLD700 dosimeters and comparison with MCNP calculations. J Med Phys. 2014;39(1):10-17.
29. Fischer HW, Tabot BE, Poppe B. Activation processes in a medical linear accelerator and spatial distribution of activation products. Phys Med Biol. 2006;51(24):N461-N466.
30. Ho L, White P, Chan E, Chan K, Ng J, Tam T. Evaluation of optimum room entry times for radiation therapists after high energy whole pelvic photon treatments. J Occup Health. 2012;54(2):131-140.
31. Lim YK. Recent trend of occupational exposure to ionizing radiation in Korea, 2015–2019. J Radiat Prot Res. 2021;46(4):213-217.
32. United Nations Scientific Committee on the Effects of Atomic Radiation. 67th session of UNSCEAR: Report of the United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR. 2021.
33. Valentin J. Protecting people against radiation exposure in the event of a radiological attack. ICRP Publication 96. Ann ICRP. 2005;35(1):1-110.
34. Lin TC, Lin CY, Li KC, Ji JH, Liang JA, Shiau AC, et al. Automated hypofractionated IMRT treatment planning for early-stage breast Cancer. Radiat Oncol. 2020;15(1):67.
35. Gibbons JP, Antolak JA, Followill DS, Huq MS, Klein EE, Lam KL, et al. Monitor unit calculations for external photon and electron beams: report of the AAPM Therapy Physics Committee Task Group No. 71. Med Phys. 2014;41(3):031501.
36. Najafi M, Deevband MR, Yousefi Diba AA, Amin Moghaddam A. Determination of room entry times for radiation therapists after routine 15 MV photon treatments. Int J Radiat Res. 2015;13(4):379-382.
Fig. 1Projection view of CPX4 (Mentor Worldwide LLD) breast tissue expander for (A) side view, (B) frontal view, and (C) subcomponents of the injection port. 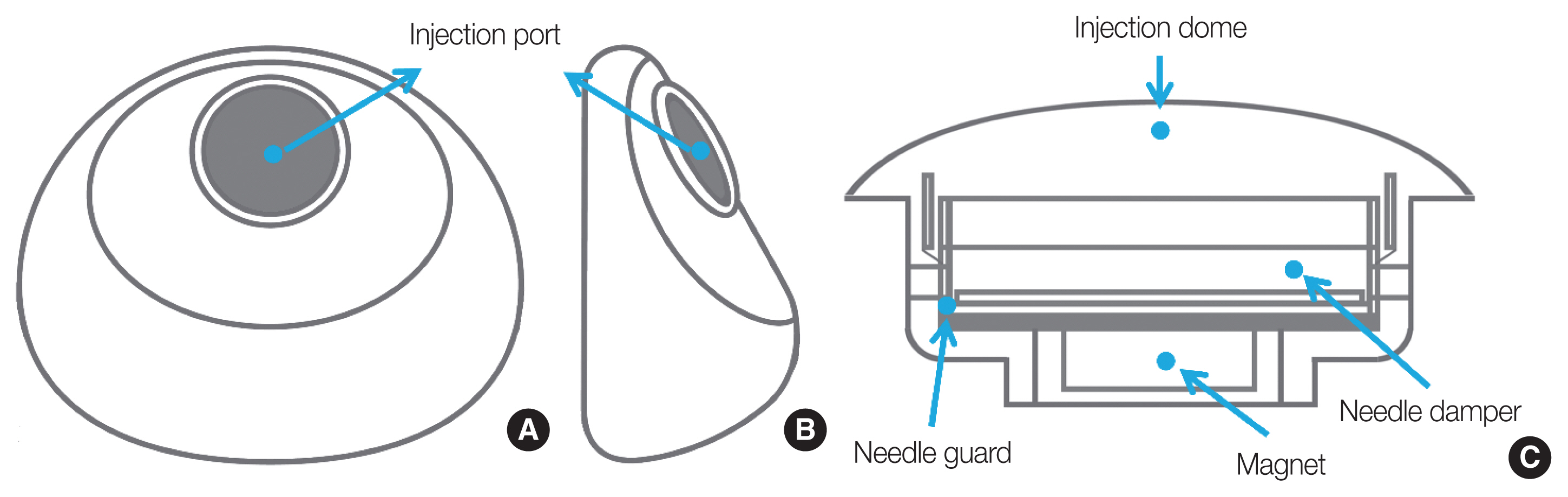
Fig. 2Experimental setup to evaluate the types of radiation and to measure instantaneous dose rates after irradiations to CPX4 (Mentor Worldwide LLD) breast tissue expander. SSD, source to surface distance; F.S., field size. 
Fig. 3Measured instantaneous dose rates (IDRs) according to the energy after 5,000 monitor units irradiation to CPX4 (Mentor Worldwide LLD) breast tissue expander. FFF, flattening filter free. 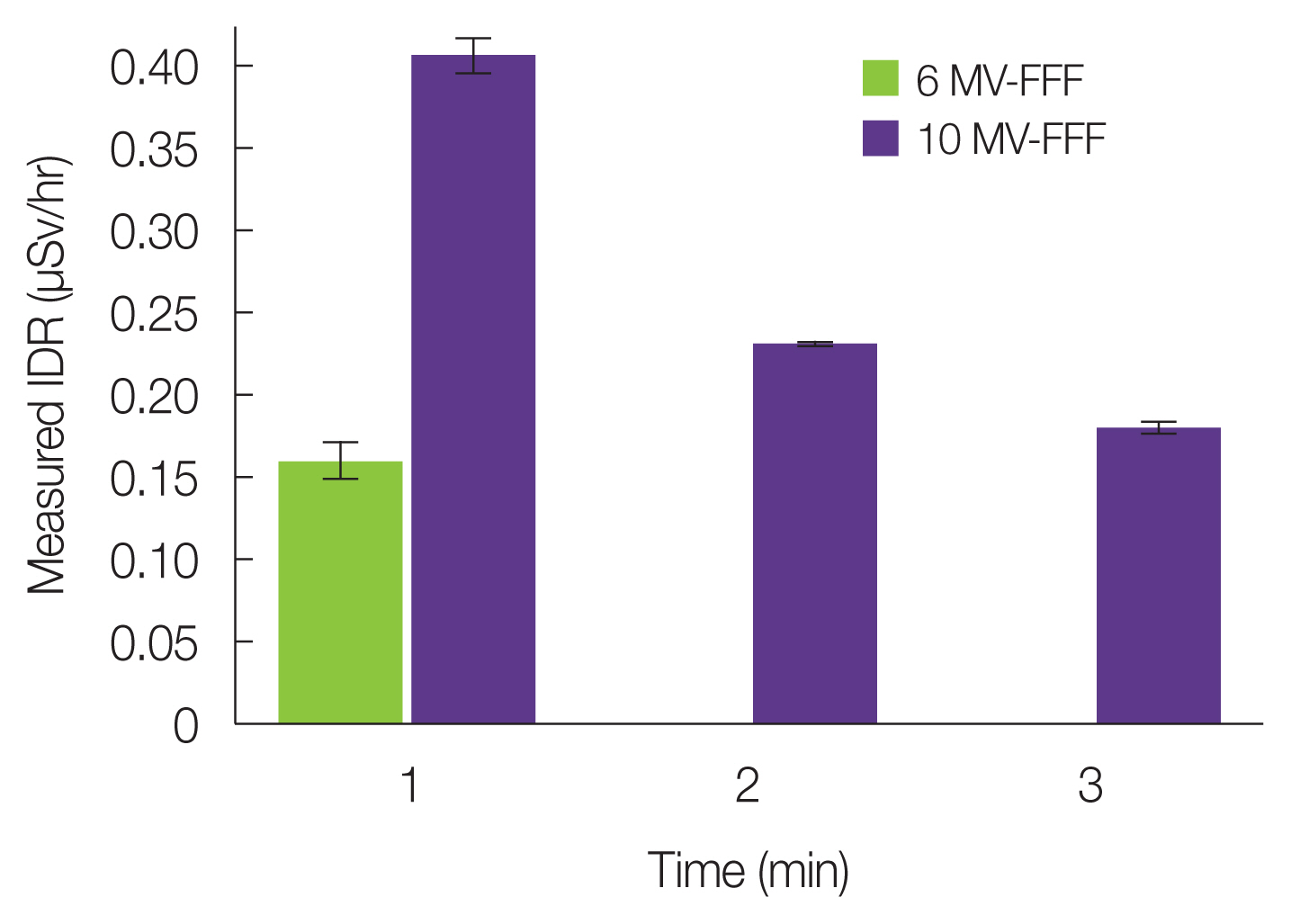
Fig. 4Three successive energy spectrum measurements after 5,000 monitor units delivery with 10 MV-flattening filter free photon beam to CPX4 (Mentor Worldwide LLD) breast tissue expander. 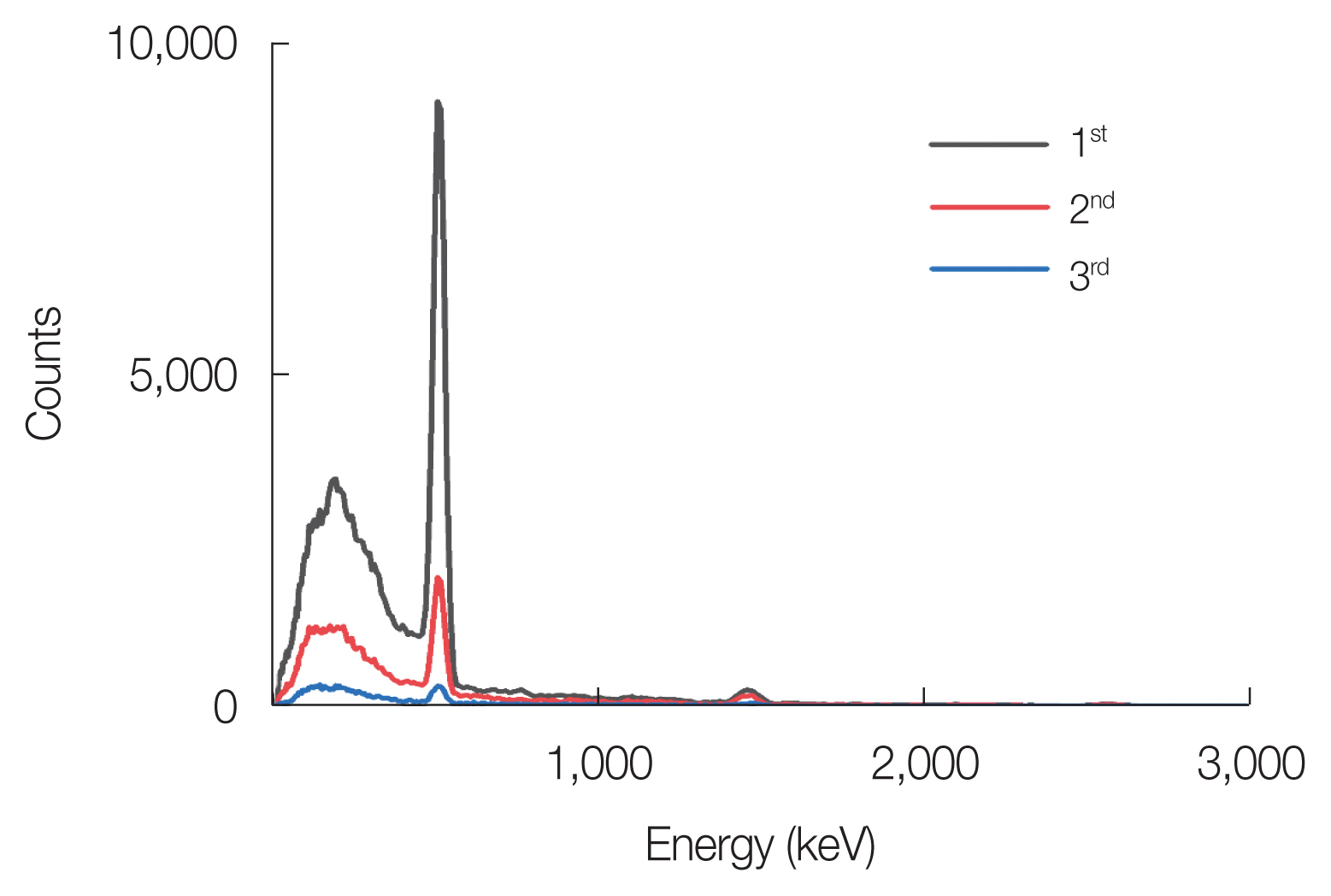
Fig. 5Measured instantaneous dose rates (IDRs) according to the machine dose rate after irradiation to CPX4 (Mentor Worldwide LLD) breast tissue expander. MU, monitor unit. 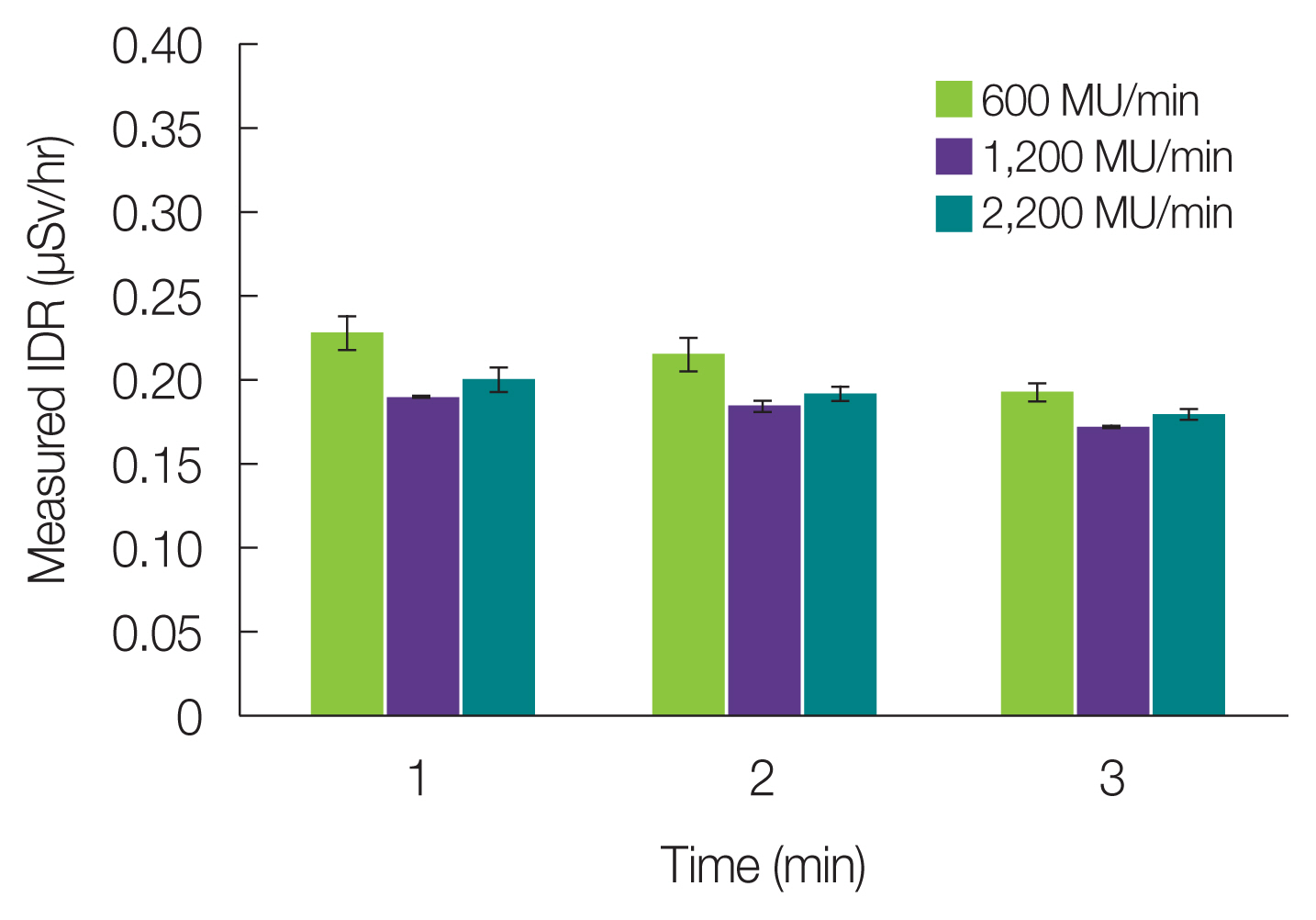
|
|
||||||||||||||||||||||||||||||||||||||