Introduction
Some of the radionuclides that had been released to the atmosphere from the Fukushima Dai-ichi Nuclear Power Plant accident were transported to the Korean territory [1]. They included such radionuclides as 134Cs, 137Cs, 131I, and 90Sr. It was not known, however, whether 90Sr had also reached to Korea or not, since 90Sr and its daughter 90Y are pure beta emitters and thus cannot be detected by gamma spectrometry. Conventional measurement of 90Sr in environmental samples involves a lengthy chemical process. 90Sr constitutes a long-term biological hazard because it accumulates in bone tissues, and has long physical and biological half-lives; 28.6 and 49.3 yr, respectively [2].
Nearly 99% of radioisotopes from atmosphere go to soil through precipitation. Absorbed by soil radioactive elements may move with soil solutions horizontally and vertically or stay in root region of plants and then enter the food chain. The process of vertical migration of radioactive fallout, downwards into soil is limited. Year velocity of migration ranges from a few to several cm and depends on the type of soil, its permeability, amount of rainfall, and type, as well as properties of the element.
Moss and lichen have been used commonly as bioindicators of fallout radionuclides [3–6]. These bioindicators have crucial advantages in determining atmospheric fallout radionuclides, such as a large surface/volume ratio, and lack of well-developed root systems. Moss has a specific surface area 10 times larger than that of herbaceous plants [7]. Another advantage is that sampling can be carried out throughout the year because of limited seasonal variations in moss and lichen morphology. Lichens grow very slowly, thus making them suitable for determining long-term deposition of fallout radionuclides.
Horwitz and his co-workers have shown that strontium may be efficiently extracted from Sr-Spec resin [8]. Nowadays, 90Sr separation from a large variety of samples like food, milk, soil, etc. by using Sr-Spec resin is reported in the literature [9–17].
The aim of this work was to get information about 90Sr contamination of the environment by using soil and moss from selected areas in Jeju Island, Korea. Additionally, we tried to improve the Sr-Spec method by searching for a procedure to get pure 90Sr spectra. This belongs in the methods available as Sr-Spec resin in prepacked columns. The elution containing 90Sr and the inactive Sr carrier was mixed with a scintillation cocktail and measured by liquid scintillation counting.
Materials and Methods
1. Materials and apparatus
All chemicals used are of analytical grade. The carrier solution Sr(NO3)2 is prepared by weighing from the corresponding salt. The soil and moss samples were selected from a collection of samples gathered from the Jeju Island. Reagents used for the digestion of the moss samples were 37% analytical grade HCl (Merck, Darmstadt, Germany) and 65% analytical grade HNO3 (Merck, Darmstadt, Germany). Sr-Spec resin (particle size: 100 μm to 150 μm) was obtained from Eichrom Industries, Inc. (Lisle, IL). A standard solution of 90Sr/90Y, provided by Korea Research Institute of Standards and Science (KRISS), was used for calibrating the counting efficiency of 90Sr and 90Y as a function of quenching. Separation of 90Sr and 90Y was carried out with extraction chromatography utilizing a Sr-Spec column. Ultima Gold LLT (Perkin Elmer, Waltham, MA) scintillation cocktail was mixed with the sample solution in a 20 mL low-potassium glass vial. Quantulus 1220 (Wallac, Turku, Finland; now Perkin Elmer, Waltham, MA) was used for the measurement of radiostrontium by liquid scintillation counting (LSC).
2. Sampling
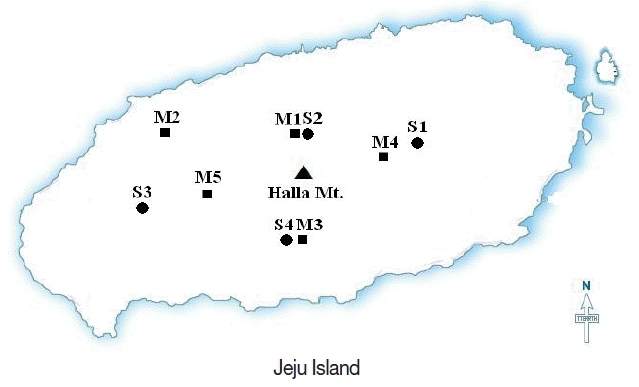
This study took place at Jeju Island, ca. 1,400 km southeast of the Fukushima Daiichi Nuclear Power Plant. Annual rainfall of Jeju Island ranges between 1,000 mm and 1,800 mm, with an average of 1,460 mm. The altitude of Mt. Halla (center place of Jeju Island) is 1,950 m. Figure 1 shows the geographic distribution of the sampling locations in Jeju Island.
The soil samples were collected from 4 locations in 2013 (Figure 1, Table 1). Sampling area and locations are presented in Table 1. The sampling locations were selected based on the following preferences: the land should be exposed to open air without any growing tree except for small grasses, and it should be flat and stable with very little disturbance induced by natural and human actions. Our soil samples stem from the eastern part (Sangumburi, 424 m), northern part (Gwaneumsa, 548 m), the western part (Dolohreum, 692 m) and the southern part (Miaksan, 472 m) of the Mt. Halla in Jeju island. At each location, the soil sample was collected from a square area of 50×50 cm after removal of organic matters such as leaves or twig pieces lying on the ground. A rectangular soil core (15×10 cm, 20 cm depth) was taken with the help of a steel coring tool. The cored sample was carefully divided into 10 depth subsamples in every 2 cm. In order to remove moisture, the soil sample was dried for 24 h in an oven kept at 105°C. The dried soil was then passed through a sieve with 2 mm holes, and pulverized for a homogeneous granularity.
The moss samples were collected from 5 locations in 2011 and 2012 (Figure 1). Sampling area and locations are presented in Table 2. The moss sample was collected by scraping it off stones or rocks existing nearby. The raw soil sample collected was weighed after removal of rubble sand organic matters to ensure that similar amounts of samples had been collected at all locations. Each moss sample was carefully cleaned from soil and other debris in the laboratory. The samples were rinsed in water twice to remove any remaining debris or soil particles. All samples were dried at 105°C for 24 hours. The samples were homogenized and powdered in the laboratory.
3. Strontium separation of soil
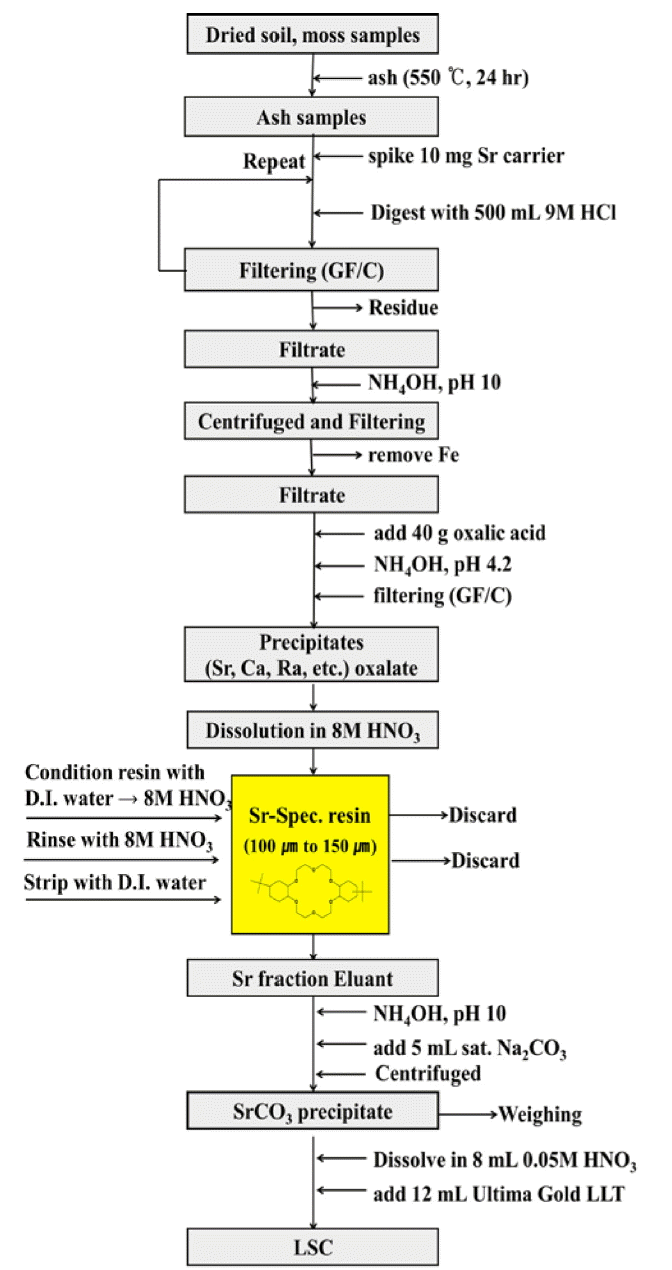
To determine the typical radiometric and gravimetric yields, carriers were spiked into the samples. The samples were then digested with HCl. The analysis flowchart is shown in Figure 2. The 100 g dried soil samples were ashed at 550°C in an electric furnace. Soil ashes were dissolved in 9 M HCl (500 mL), and 10.0 mg·mL−1 Sr carrier (1.0 mL) was added. After stirring for 5 h, the sample solution was filtered; the beaker and filter were washed with 9 M HCl (500 mL) and a few milliliters of hot water. Iron precipitate was removed from the alkaline solution (>pH 10) by conc. NH4OH. A few milliliters of HCl (<pH 1) and oxalic acid (40 g) were added to the solution. Strontium oxalate was precipitated by conc. NH4OH (ca. pH 4.2). The precipitate was filtered through a GF/C filter paper. The precipitate was dissolved in 8 M HNO3 (ca. 30 mL). Subsequently 90Sr was purified on Sr-Spec resin. After washing with of distilled water (50 mL), the Sr-spec column was preconditioned with 8.0 M HNO3 (20 mL). Then the sample solution was loaded onto the column. After rinsing with 20 mL of 8.0 M HNO3 solution, strontium was stripped with 20.0 mL of distilled water (or 0.05 M HNO3) and collected in a glass vial. The flow rate for all procedures was kept at 1 mL·min−1.
4. Strontium separation of moss
The whole of each sample was ashed at 550°C in an electric furnace. The mass of ashes from the samples was in the range 5 to 20 g depending on sample size. Moss ashes were dissolved in 250 mL of 9 M HCl and 0.1 mL of 10.0 mg·mL−1 Sr carrier was added. After stirring for 5 hours, the sample solution was filtered; the beaker and filter were washed with a few milliliters of 9 M HCl. 20 g of oxalic acid was added to the solution. Strontium oxalate was precipitated by conc. NH4OH (ca. pH 4.2). The precipitate was filtered through a GF/C filter paper.
The precipitate was dissolved in 8 M HNO3 (ca. 30 mL). Subsequently 90Sr was purified on Sr-Spec resin. After washing with of distilled water (50 mL), the Sr-spec column was preconditioned with 8.0 M HNO3 (20 mL). Then the sample solution was loaded onto the column. After rinsing with 20 mL of 8.0 M HNO3 solution, strontium was stripped with 20.0 mL of distilled water (or 0.05 M HNO3) and collected in a glass vial. The flow rate for all procedures was kept at 1 mL·min−1.
5. Radioactivity determination
A saturation Na2CO3 (5 mL) was added to the stripped solution of Sr-Spec resin in the alkaline solution (>pH 10), and then allowed to stand at 50°C until a white precipitate (SrCO3) separated. This was collected by centrifugation, and thoroughly dried in vacuo. The Sr yield of the respective soil sample was measured by gravimetric method. The dried sample was dissolved in 8.0 mL of 0.05 M HNO3. Then 8.0 mL of this solution transferred to 20 mL low-potassium glass vial and 12 mL of Ultima Gold LLT scintillation cocktail; to make a total volume of 20 mL. The vial was shaken vigorously and immediately counted by LSC on a Quantulus1220. The lower limit of detection (LLD) of 90Sr was calculated according to [18]. It was 0.1 Bq·kg−1 for soil samples (counting times and chemical recovery as with ash samples, sample mass: 100 g of dried soil).
To determine the activity of radiostrontium of a sample, the counting efficiency of the radiostrontium isotope with a quench level must be measured. This was done by counting a quench series of reference standards for each isotope. Five milliliter of 90Sr (27.96 Bq·mL−1) standard solution was added in a teflon vessel and evaporated to near dryness by heating on a hot plate at 150°C. The dried standard was dissolved in 10.0 mL of 8.0 M HNO3, then loaded onto a Sr-spec column prepared as for the samples. After washing with 30 mL of 8.0 M HNO3 solution, strontium was stripped with 5×4 mL of distilled water (or 0.05 M HNO3) and collected in a glass vial. The flow rate for all procedures was kept at 1 mL·min−1. The solution evaporated by heating on a hot plate at 100°C. The dried sample was dissolved in 10.0 mL of 0.05 M HNO3. Then 2.0 mL of this solution transferred to five 20 mL low-potassium glass vial and 18 mL of Ultima Gold scintillation cocktail and nitromethane (0 μL, 150 μL, 300 μL, 450 μL, 600 μL) of quenching agent were added, to make a total volume of 20 mL. The vial was shaken vigorously and immediately counted by LSC.
Results and Discussion
1. 90Sr activity concentrations of soil
The 90Sr activity concentrations of soil samples are shown in Table 3. The 90Sr vertical concentrations in the investigated soil samples were 18.24±0.42–2.77±0.22 Bq·kg−1 in eastern part (S1; Sangumburi, 424 m), 18.27±0.28–1.69±0.11 Bq·kg−1 in northern part (S2; Gwaneumsa, 548 m), 45.27±2.60–3.76±0.19 Bq·kg−1 in the western part (S3; Dolohreum, 692 m) and 8.70±0.59–1.09±0.14 Bq·kg−1 in the southern part (S4; Miaksan, 472 m) of the Mt. Halla in Jeju island, respectively.
Activities of 90Sr show the highest value at the surface soil and decrease with depth (Figure 3).
2. 90Sr activity concentrations of moss
Moss samples were measured immediately after extraction, one week later and finally after two weeks. Samples were spiked prior to digestion to correct for losses of analytes during sample preparation procedure. The Sr concentrations in the investigated mosses samples were 363±3.0 Bq·kg−1, 97.7±1.0 Bq·kg−1, 91.7±0.7 Bq·kg−1, 79.6±0.8 Bq·kg−1, and 225±2 Bq·kg−1 (-dry weight), respectively. The chemical yield and the 90Sr activity concentration of each sample are shown in Table 4. The measured 90Sr concentration is higher at high-altitude locations.
Conclusions
In this study, we determined 90Sr concentrations in soil and moss of Jeju Island (Korea) using strontium specific ion exchange columns in combination with liquid scintillation spectrometry. A large amount of airborne radioactive species had been released following the Fukushima Daiichi nuclear reactor accident. We present an efficient method of measuring the 90Sr activity in environmental samples, which is based on using ion exchange columns loaded with a Sr-Spec resin and liquid scintillation spectrometry. The activities of 90Sr in soil and moss samples were investigated at nine locations of Jeju Island, Korea. The 90Sr vertical concentrations in the investigated soil samples were 18.24 to 2.77 Bq·kg−1 in eastern part, 18.27 to 1.69 Bq·kg−1 in northern part, 13.46 to 3.76 Bq·kg−1 in the western part and 8.70 to 1.09 Bq·kg−1 in the southern part of the Mt. Halla in Jeju island, respectively. Activities of 90Sr show the highest value at the surface soil and decrease with depth. The measured activity concentrations were between 79.6 Bq·kg−1 -dry (moss in Jeju racehorse Fostrering Farm site) to 363 Bq·kg−1 -dry (moss in Gwaneumsa site). The result will be first study of the activity of 90Sr in moss samples from Jeju Island, Korea.