Introduction
The skin, comprising the epidermis and the vascularized dermis, provides a vital protective barrier against environmental insults and is one of the most complex and reactive organs in the body. The increasing use of radioactive materials in industry, medicine, science, and the military as well as in localized areas of high radiation within nuclear facilities have significantly increased the potential of large-scale, uncontrolled exposure to radiation [1]. Radiation-induced skin injury is a common side-effect of radiation exposure in therapeutic applications and is also the most common problem in radiation accidents [2]. These radiation skin toxicities can negatively affect the quality of a patientŌĆÖs life in terms of the pain associated with the injury as well as the premature termination of radiation treatment [3]. Radiation-induced skin injury begins with erythema, followed as the dose increases by dry and moist desquamation. Chronic radiation skin injury also results in delayed ulcers, fibrosis, and telangiectasis [4]. Although these symptoms of radiation-induced skin damage have been described, the biological mechanism underlying these effects has not yet been studied [2, 5].
The effects of radiation on tissues vary depending on the radiation type. Gamma rays are used in a wide variety of medical applications such as CT scans and radiation therapy because they exert enough energy to penetrate tissues. Beta decay results from the emission of an electron (or negative beta particle) from a nucleus. Although ╬▓-rays have weak transmission power, they have sufficient energy to penetrate the skin. ╬▓-rays may therefore be an external radiation risk [6]. The biological effects of ╬▓-irradiation in normal tissues and tumors are of interest for clinical radiotherapy, for radiation protection purposes, as well as for a greater understanding of radiation-induced inactivation of cells for different radiation types [7]. The effects of beta radiation on the skin, however, have not been well studied.
The pig has been widely used as an experimental animal in biomedical research for many centuries. The physiological and anatomical similarities between humans and pigs have deemed this animal an appropriate model for humans in many research areas [8]. Human and porcine skin tissues have microscopically heterogeneous structures that are similar in their morphology, cellular composition, and physiological properties [9]. Porcine skin has been used in drug delivery studies, to assess wound healing after heat or chemical burning, as well as to model light distribution in the skin [10].
In this study, a minipig model was used to compare the effects of ╬▓-rays from 166Ho and ╬│-rays from 60Co on the skin.
Materials and Methods
1. Animals
Six-month-old male G├Čttingen minipigs (mean weight, 19 kg; range 18ŌĆō20 kg), which were obtained from the PWG genetics (Seoul, Korea), were used in this experiment. Two minipigs were fed a standard animal diet. All animal experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the Korea Institute Radiological and Medical Sciences (KIRAMS).
2. Irradiation and sample biopsy
To assess the effects of ╬│- and ╬▓-rays on the minipig skin, irradiation was carried out on the flank skin of minipigs. For all procedures the animals were anesthetized with tiletamine/zolazepam (Zoletil 50┬«, Virak Korea, Seoul, Korea) and medetomidine (Domitol┬«, Pfizer Animal Health Korea). To observe the skin effect of radiation, we used the right and left font back lesion of dorsal skin. We evaluated clinical status scoring in the right flank and skin biopsy performed in the left flank. Three to four days prior to irradiation, the hair in the treatment area on each pig was clipped and the position of the fields of interest was marked and tattooed with India ink. The fields were gamma irradiated with a range of 50 Gy using a 60Co ╬│-rays irradiation therapy unit (Theratron 780, AECL, Ontario, Canada) at a dose-rate of 130.1 cGy┬ĘminŌłÆ1 (field size: 5 cm, source-to-surface distance: 80 cm, depth: 1 cm [bolus 1 cm]). Each pig received 50 Gy irradiation.
To assess the effects of ╬▓ emission (166Ho) on the minipig skin, irradiation was carried out on the dorsal skin of mini-pigs. 165Ho(NO3)3 5H2O (231 g) and 346 mL tetrahydrofuran (Merck, M├╝nchen, Germany) were completely dissolved in a solvent mixture of 235 mL dimethylformamide (Merck), 2 L tetrahydrofuran (Merck), and 462 g polyurethane (Dow chemicals, Midland, MI) at room temperature. The solution was uniformly affixed to adhesive tape (approximately 500 ╬╝m in thickness). Patches of the treated tape were assailed in a nuclear reactor at a neutron flux of 1├Ś1013 n┬ĘcmŌłÆ2┬ĘsecŌłÆ1 to convert 165Ho to 166Ho, which is a ╬▓ emitter (Emax=1.84 MeV) with a half-life of 26.9 hours. 166Ho also emits gamma photons (5.4% of 0.081 MeV and 0.9% of 1.38 MeV). To assess the effects of ╬▓ emission (166Ho) on the minipig skin, irradiation was carried out by applying the Holmium patches on the skin of minipigs. Six pieces of 1├Ś7 cm-sized rectangular patches were separately applied on the dorsal skin surface and were firmly affixed with adhesive tape for 23 minutes for a total surface radiation dose of 50 Gy. At the time of irradiation, three skin biopsies were taken and pinned to cork to maintain the original size. A 5 mm punch biopsy was taken under anesthesia from the irradiated skin area and the corresponding skin area of the healthy skin. Samples of the biopsy were processed for embedding in paraffin wax after fixation in 10% buffered formalin.
3. Examination of clinicopathological alterations in gross appearance
The skin of the minipigs was carefully evaluated for 12 weeks after irradiation and the observed skin reactions were scored using a clinical status scoring system. Irradiated lesions were assessed for skin reactions and operation scars were examined. The following scoring system was used to measure radiation acute reactions in the skin: Grade 0, normal; Grade 10ŌĆō30, slight swelling, edema, and erythema; Grade 40ŌĆō60, dry desquamation and mild early moist desquamation changes; Grade 70ŌĆō90, severe moist desquamation with some necrosis; and Grade 100, severe necrosis and loss of epidermis [5, 13].
4. Histological examination
For analysis by light microscopy, tissue samples of the skin biopsies of each field that were fixed in 10% buffered formalin, dehydrated in grade ethanol, and embedded in paraffin were assessed. The tissue sections were cut to 4 ╬╝m thickness on a rotary microtome (Leica Ultracut, Leica Microsystems, Wetzlar, Germany), mounted on clean glass slides, cleared, hydrated, and finally stained with haematoxylin and eosin (H&E) for histological evaluation of skin injury, apoptotic cells, epidermal thickness, density of basal cells, and density of epithelial cells according to standard protocols. The slides were labeled using codes to prevent observer bias during evaluation. All tissue sections were examined by microscopy for characterization of histopathological changes. Photographs taken from skin samples were digitized using a digital camera mounted on a microscope (Leica DM IRBE, Leica Microsystems GmbH, Wetzlar, Germany). The resulting images were analyzed using image analysis software (Leica QWin, Leica Microsystems, Wetzlar, Germany).
The longest rete ridge on each slide was measured from the bottom of the basal layer to the bottom of the stratum corneum, avoiding areas where the inclusion seemed to be oblique. Means were calculated from the measurements for each slide. The same method was adopted to measure the suprapapillary thickness.
Cell density in the basal layers was determined by counting cells along a minimum of 5 mm of basement membrane. Results were expressed as the count per millimeter length of basement membrane. The degenerate cells, i.e., those cells exhibiting pycnosis and shrinkage necrosis, were excluded from these calculations.
5. Immunohistochemical study
Fixed tissues were processed in paraffin, cut into 4 ╬╝m-thick coronal sections, and deparaffinized. The sections were incubated with 5% normal goat serum for 60 minutes and then with rabbit anti-mouse cyclooxygenase (COX)-2 (18-7379, 1:200 dilution; Zymed) in PBS-T overnight at 4┬░C. The sections were then reacted with biotinylated goat anti-rabbit IgG. Immunoreactivity was then assessed using the avidin-biotin peroxidase complex (Vectastatin Elite ABC kit). The peroxidase reaction was developed using a diaminobenzidine substrate kit (DAB Substrate Kit SK-4100; Vector Laboratories). As a control, the primary antibodies were omitted for a few test sections in each experiment. The sections were counterstained with hematoxylin before being mounted and examined by microscopy.
6. Statistical analysis
The data are reported as the mean┬▒standard error of the mean (SEM), and data analysis was performed using one-way analysis of variance (ANOVA) followed by a StudentŌĆōNewmanŌĆōKeuls post hoc test for multiple comparisons. In all cases, a p-value <0.05 was considered significant.
Results and Discussion
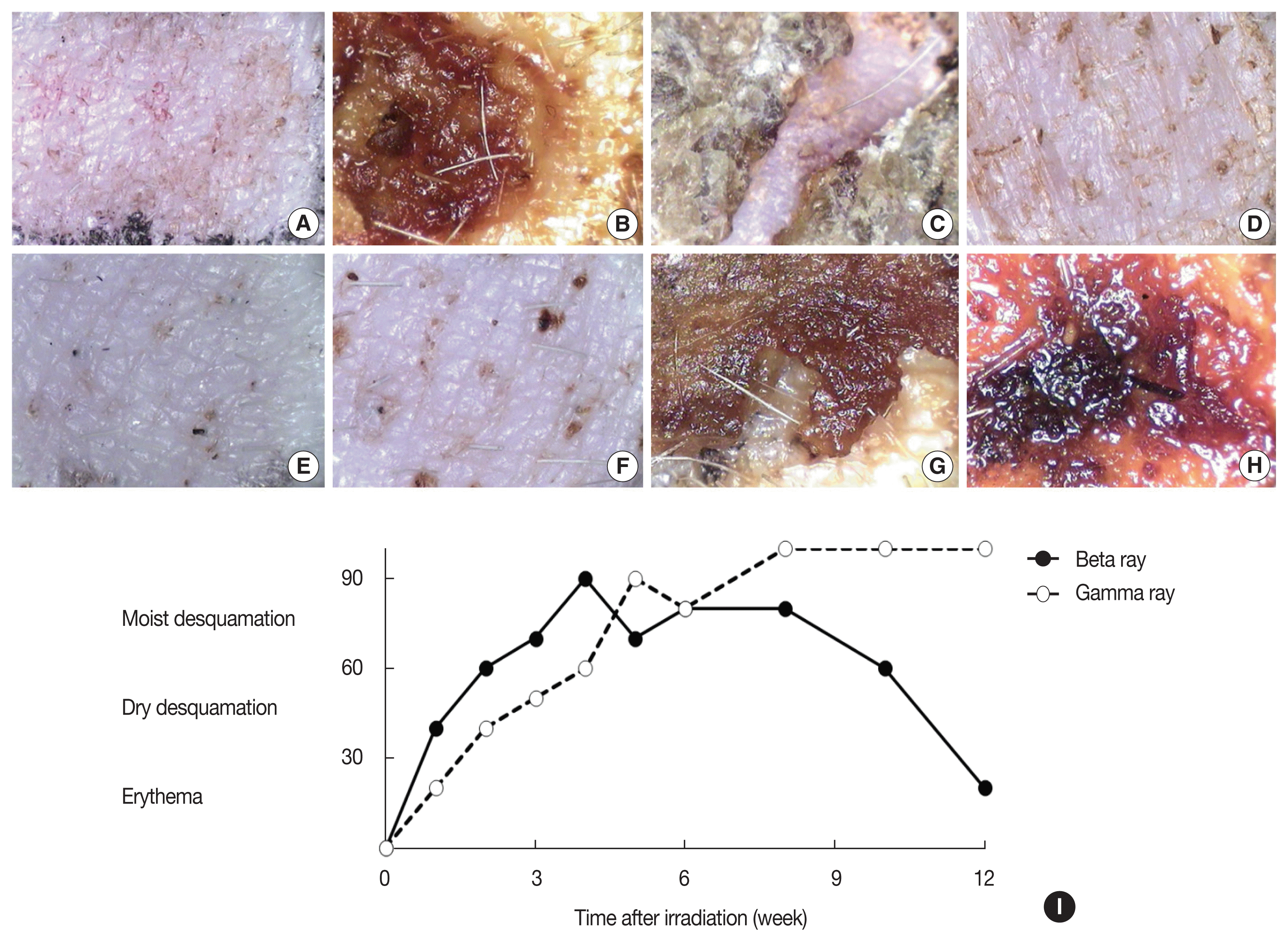
Time-dependent changes in skin reactions after ╬▓- and ╬│- irradiation of 50 Gy are summarized in Figure 1. The range of acute change begins with erythema. Over time, pigmentation, epilation, and desquamation appear in irradiated skin lesion. Between the two radiation types, ╬▓-irradiation was shown to exhibit more severe skin injury (Figure 1A and 1B) than ╬│-irradiation at 1ŌĆō4 weeks after irradiation (Figure 1E and 1F). At eight weeks post-irradiation, the ╬▓-irradiated skin lesion had gradually repaired (Figure 1C and 1D); however, the ╬│-irradiated skin had not recovered and progressed to necrosis (Figure 1G and 1H).
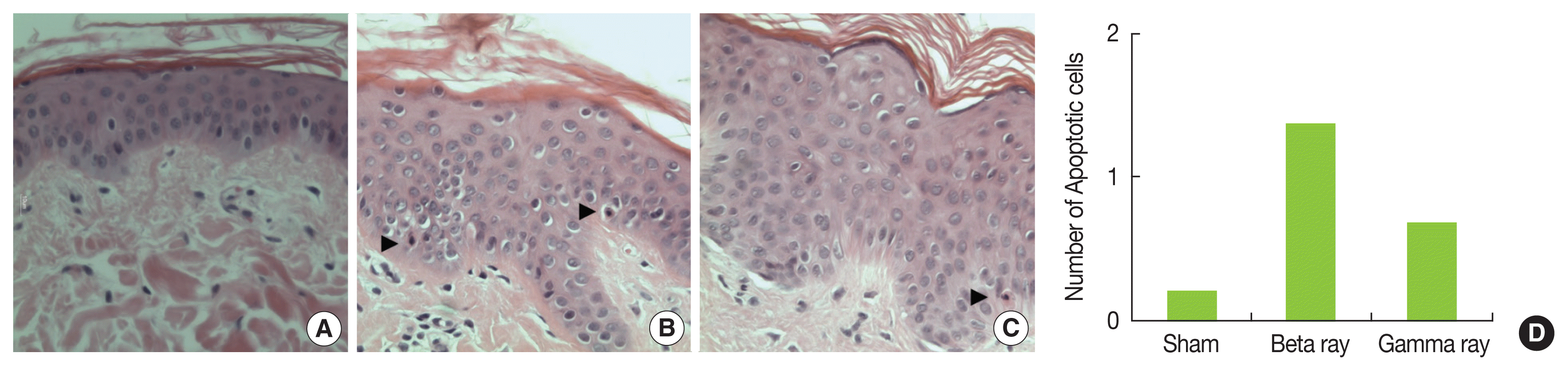
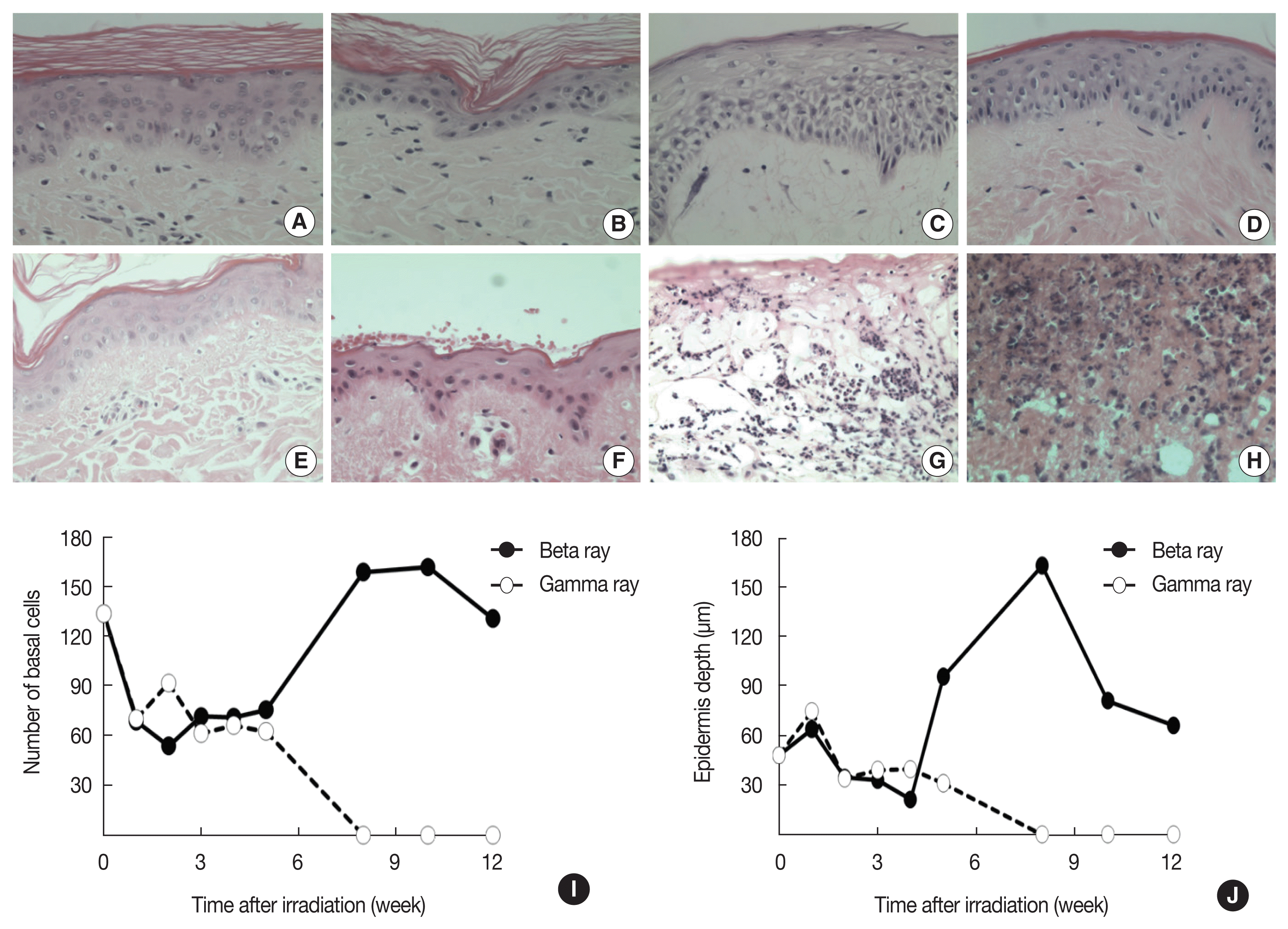
Apoptotic cells were easily recognized based on the condensation of the cytoplasm and nuclear chromatin in these cells. Dead cells break up into several fragments and not all fragments necessarily contain nuclear material. These cytoplasmic fragments can usually be recognized by their eosinophilic staining properties in H&E staining [11]. The apoptotic cell fraction was 2-fold higher in the ╬▓-irradiated skin compared with the ╬│-irradiated skin at 7 days post-irradiation (Figure 2D) and apoptotic cells were mainly detected in the basal layer compared with the granular layer. A small number of cells in the basal layer exhibited apoptosis and the maximal frequency was observed at 7 days after irradiation (Figure 2AŌĆō2C). In H&E staining, demonstrated ╬▓-rays-induced desquamation of the epidermis until 4 weeks post-irradiation; however, this effect was not observed in the dermal layer (Figure 3AŌĆō3D). Epidermal detachment and accumulation of inflammatory cells in the dermis of gamma-irradiated lesions were observed (Figure 3EŌĆō3H) and the alterations in basal cell density and epidermal depth were in accordance with gross changes (Figure 3I and 3J). ╬▓-irradiation-induced skin lesions exhibited diminished basal cell density and epidermal depth earlier than the lesions induced by ╬│-irradiation at 1ŌĆō4 weeks after radiation; however, basal cell density and epidermal depth in the ╬▓-irradiated skin increased sharply to higher than control levels at 6ŌĆō8 weeks post-irradiation and then gradually returned to control levels thereafter. The ╬│-irradiated skin, on the other hand, did not exhibit signs of repair (Figure 3C, 3D, 3G, and 3H).
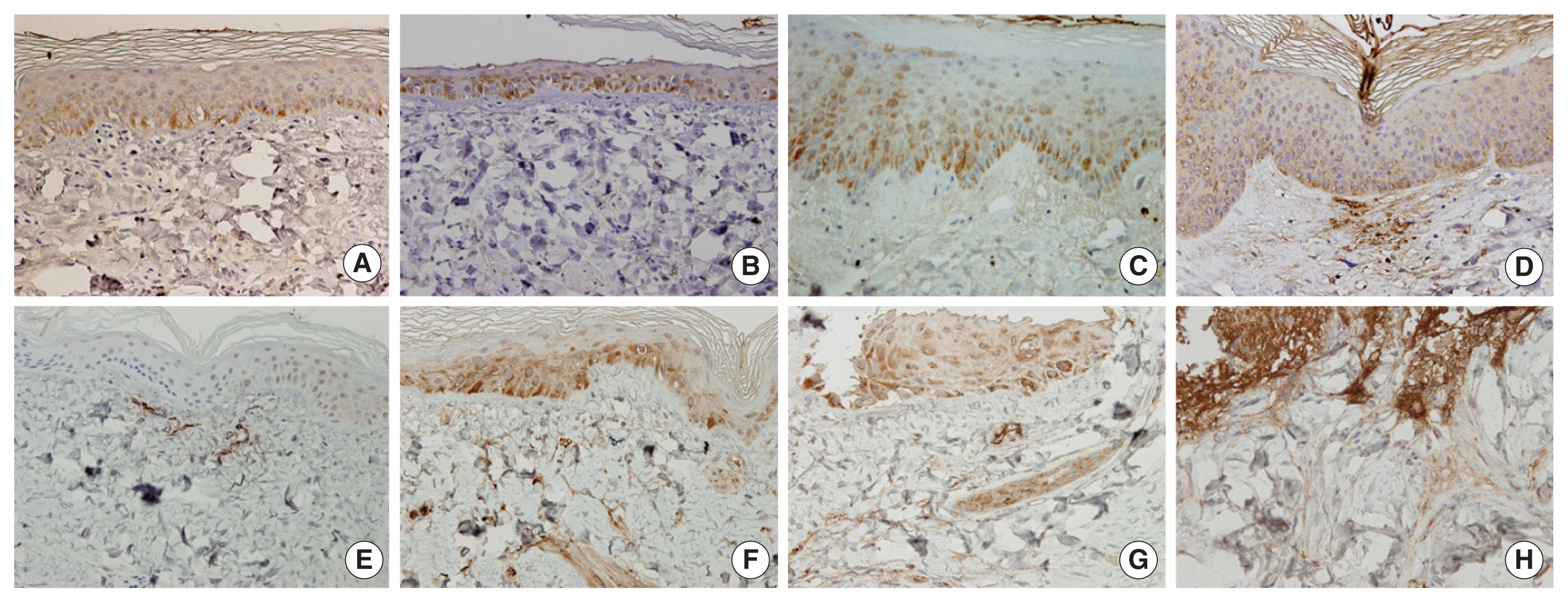
In normal pig skin, COX-2 staining was shown to be minimal with some staining in the sebaceous glands and subcutaneous tissue, while there was no staining in the epidermis (Figure 4A). In the ╬│-irradiated skin, COX-2 was expressed not only in the epidermis but also in endothelial cells and fibroblasts. This effect increased with increasing severity of damage (Figure 4EŌĆō4H). The ╬│-irradiated skin lesions were characterized by the expression of COX-2 in the vessels and nerves of the dermis and this staining pattern increased in over time. In the ╬▓-irradiated samples, transient COX-2 expression was only observed in the epidermis (Figure 4AŌĆō4D). By 1 week post-irradiation, the expression of COX-2 was mostly limited to the stratum granulosum in the epidermis (Figure 4A). By weeks 6ŌĆō10, increased expression of COX-2 was observed in all epidermal layers (Figure 4C) and was particularly prominent in the basal layer underlying areas of epidermal hyperplasia. The expression of COX-2 was shown to decrease as skin injury recovered at 10ŌĆō12 weeks after irradiation (Figure 4D and Table 1).
166Ho may be an ideal radioisotope as it emits high-energy ╬▓-rays (Emax=1.84 MeV) with a maximum soft tissue range of 8.7 mm. As much as 90% of the ╬▓-energy from 166HO is deposited within 2.1 mm of the skin (upper and mid dermal level of the skin) [12]. This rapid dose reduction over penetration distance would result in significantly higher doses being applied to the cells in the upper viable layer of the epidermis than to the basal layer. In particular the potential stem cells, which are largely distributed towards the bases of the rete ridges, would receive far lower doses of radiation than the cells in the upper layers [3].
The time-course of the development of gross changes in response to irradiation as well as the differences in these time courses between the different irradiation types reflect the evolution and time course of changes in the component populations of the epidermis, dermis, and microvasculature of the skin [5]. The range of acute changes begins with erythema and as time passes, pigmentation, epilation, and desquamation appear in the skin lesions. The skin was usually found to develop erythema within 1 week after ╬▓-rays treatment and this reaction worsened until 4 weeks post-irradiation as evidenced by dusky/mauve erythema. After 4 weeks post-╬▓-rays irradiation, the clinical symptoms gradually disappeared and recovered [13]. The ╬│-ray effects were found to be similar to the ╬▓-ray effects in the early stages after exposure; however, ╬│-irradiation-induced injury was not repaired and instead progressed to necrosis [13ŌĆō15].
The application of ╬▓- or ╬│-rays to skin generally appears to trigger a linear loss of basal cells [13ŌĆō16] and histological studies have demonstrated an early pyknosis of cells in the basal layers of the epidermis 7 days after irradiation, which in turn initiates an apoptotic reaction and the subsequent disruption of the total epidermis. In a previous study we showed that apoptotic cells were present at a higher frequency in the basal layer of irradiated skin than in the basal layer of normal skin and that the frequency was irradiation dose-dependent [15]. In this study, we showed that ╬▓-irradiated skin contains a higher proportion of pyknotic cells than ╬│-irradiated skin.
Changes in basal cell density and epidermal depth were previously shown to correspond to gross skin morphology scores [13ŌĆō16]. Epidermal thickness began to decrease 1 week after radiation-induced skin injury, which was evident 6ŌĆō8 weeks after ╬▓- or ╬│-irradiation. The ╬▓-rays have a faster onset of erythema and moist desquamation after exposure to radiation, while beta radiation- and not gamma radiation-induced injury was followed by a pronounced degree of recovery. Gamma-rays penetrate the whole skin layer, causing damage to blood vessels and lipids in the dermis and epidermal layer. This damage leads to erosion and ulcers, where the disappearance of the epidermal layer results from the invasion of secondary acute inflammatory cells [15].
The differences in recovery patterns between the two radiation types relates to interactions between keratinocytes and fibroblasts: keratinocytes stimulate fibroblasts to synthesize growth factors such as keratinocyte growth factor, hepatocyte growth factor, and transforming growth factor beta, which in turn stimulate keratinocyte proliferation in a double paracrine manner [17]. Accordingly, ╬│-irradiation-induced injury was delayed due to the damage to the dermal layer containing fibroblasts and endothelial cells disrupting in the interaction of keratinocytes and fibroblasts.
In this study, sequential skin tissue samples were obtained from the same individual, which allowed for continuous clinicopathological changes including the potential role of COX-2 in radiation-induced skin damage to be monitored. COX-2, a stress response protein, is known to produce the synthesis of prostaglandins involved in the inflammatory cascade resulting in tissue injury [18, 19]. In radiation-associated mucositis, the role of COX-2 has been reported to increase the severity of mucosal injury and to prolong the inflammatory and ulcerative event [19]. COX-2 expression in the epidermis is furthermore known to be induced by stimuli that result in transient hyperplasia, such as UV irradiation, including in UV-induced skin cancers and other malignancies [17]. The expression of COX-2 in radiation-induced skin damage due to high-dose ionizing irradiation has not yet been reported. Although this study does not confirm the role of COX-2 in the irradiated skin, COX-2 expression was shown here to be one of the irradiation-induced skin injury markers. In this study, COX-2 was shown to be expressed not only in the epidermis but also in endothelial cells and in fibroblasts in the ╬│-irradiated skin, where the expression was shown to increase with increasing severity of damage. In this study, ╬│-irradiation-induced skin lesions were characterized by COX-2 expression in the vessels and nerves of the dermis and lipids and the ╬│-irradiated skin of minipigs were shown to exhibit increasing COX-2 expression over time. The skin of minipigs irradiated with ╬▓-rays was shown to exhibit only transient COX-2 expression and only in the epidermis.
Conclusion
In this study, the biological effects of ╬▓- and ╬│-irradiation on the skin of minipigs were assessed and compared. Overall, ╬▓-rays were shown to induce more severe skin injury than ╬│-rays; however, the ╬▓-rays-induced injury was largely repaired over time, while the ╬│-rays-induced injury was not repaired and instead progressed to necrosis. These findings suggest that ╬│-irradiation affects the deep dermis and subcutaneous fat due to its penetrating ability, whereas ╬▓-rays irradiation induces changes only at the level of the epidermis and the upper dermis. The use of the minipig in this study demonstrates the use of the animal model as an experimental human skin model with which radiation-induced skin damage can be studied and thus as a model with which the efficacy of therapeutics to reduce the skin damage and to accelerate repair can be developed.